This kit utilizes a four-plasmid system (including the packaging plasmids gag/pol and rev, the envelope plasmid VSV-G, and the shuttle plasmid), and is designed with enhanced safety. The key innovation of this product lies in using suspended 293T cells for lentivirus packaging. Hillgene’s proprietary serum-free suspension culture technology, combined with the HiLenti® transfection reagent, enables the production of highly functional lentivirus in serum-free conditions, making it more suitable for scalable virus production with improved yields and reliability.

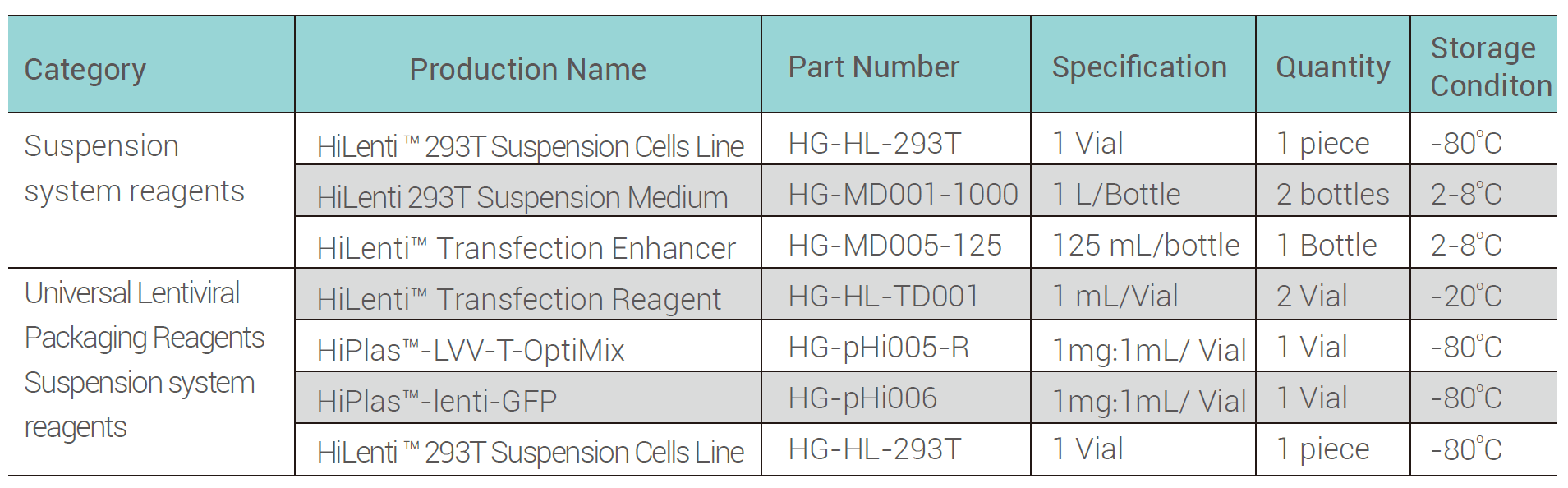
Scenarios for use
·Lentiviral packaging suitable for CAR molecular screening phase
·Lentiviral preparation suitable for CART cell testing phase
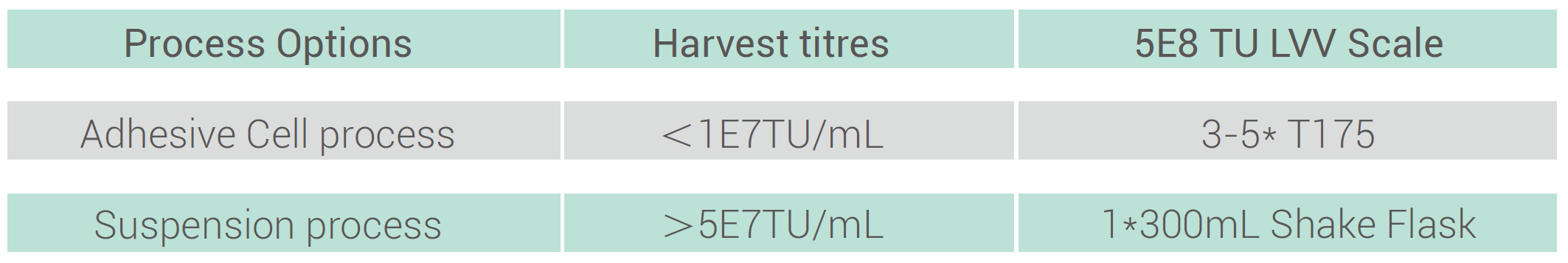
Performance indicators
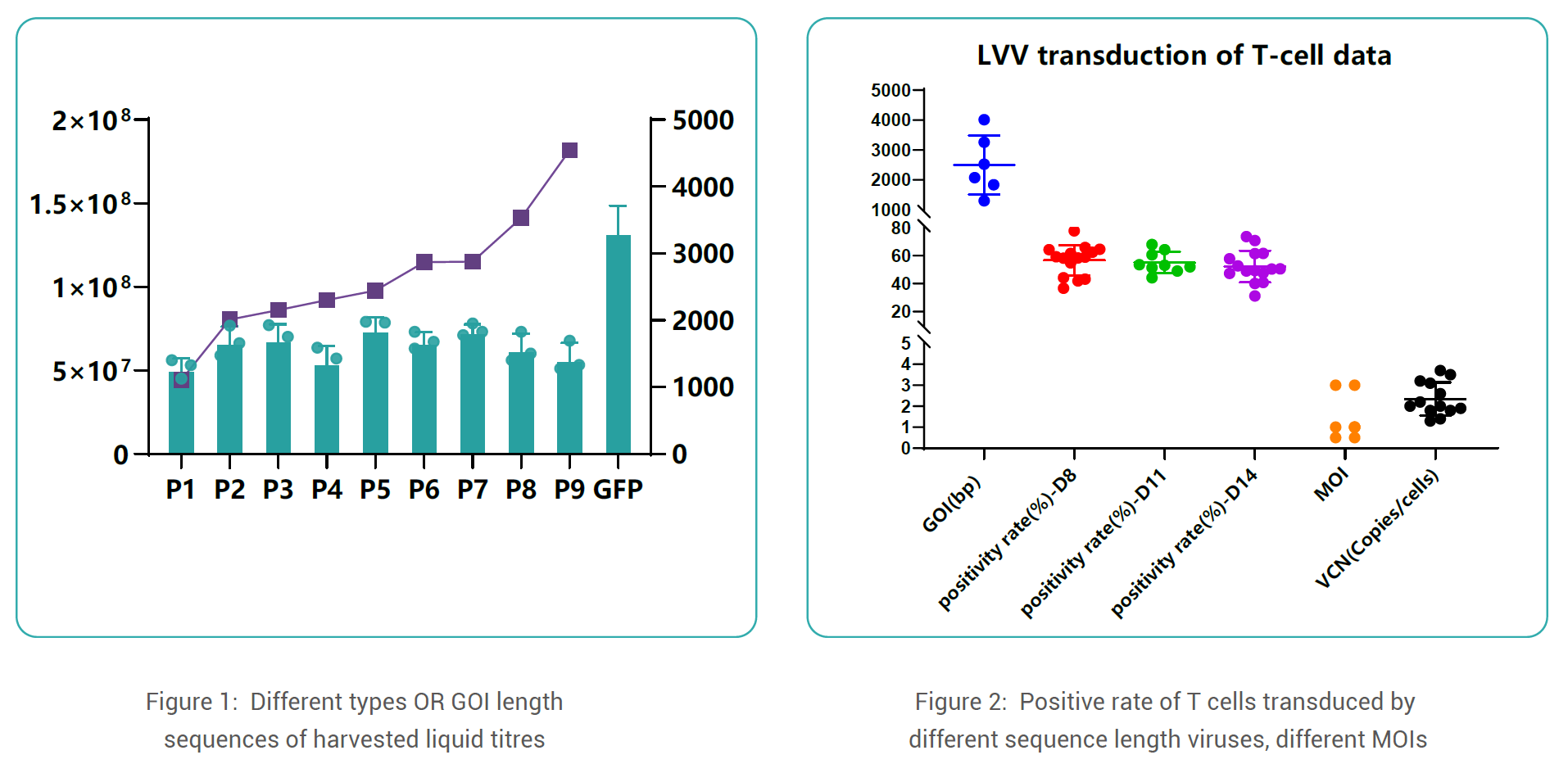
Data Display

Building 4, Yuewang Wisdom Valley, 1463 Wuzhong Avenue, Wuzhong District, Suzhou, China