Cell Cytotoxicity is a critical quality attribute (CQA) for the biological activity of immune cell therapy products (e.g., NK cells, CAR-NK cells, Tm cells, CAR-T or TCR-T cells, etc.), and it is an important element of product quality research and quality control. Compared with cell cytotoxicity methods such as lactate dehydrogenase (LDH) assay, 51Cr release assay, luciferase reporter gene assay, RTCA assay, the detection of CFSE- and 7-AAD-labelled cell killing by flow cytometry is safer, more convenient, faster and more stable.

Application
·It is suitable for the evaluation of the killing function of immune cell products such as NK cells, CAR-NK cells, CAR-T cells, TCR-T cells, Tm cells, etc. on different tumour cell lines (adherent target cells).
·CMC and quality research phase: In the early development phase of cell therapy products, the cytotoxic effect is evaluated and the process is optimised.
·Release testing, stability and comparability studies phase: During the mid-term process lock phase of cell therapy products, a cell killing method for the product is established, and release testing and stability testing of the cell product are performe.
Performance Index
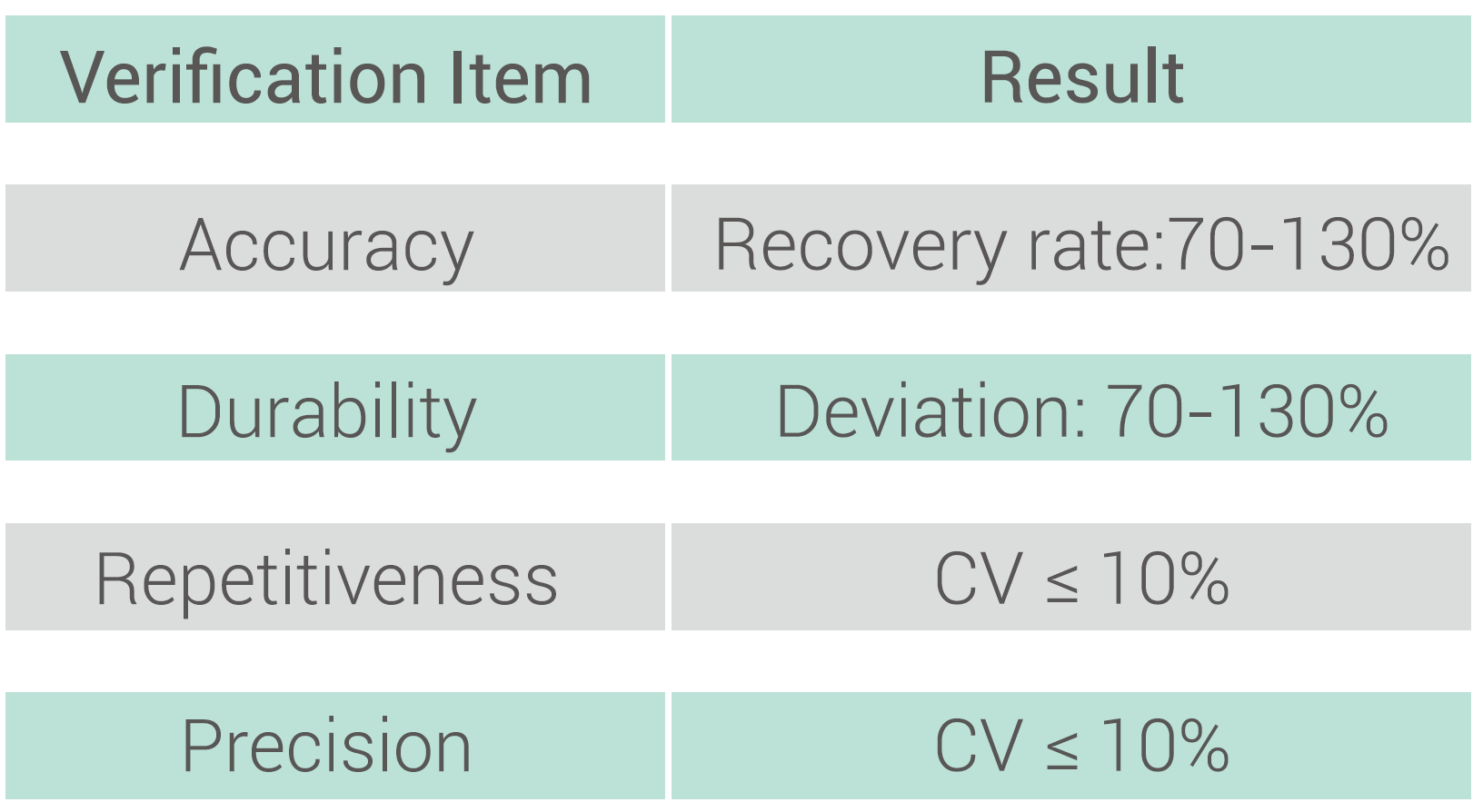
Data Sharing
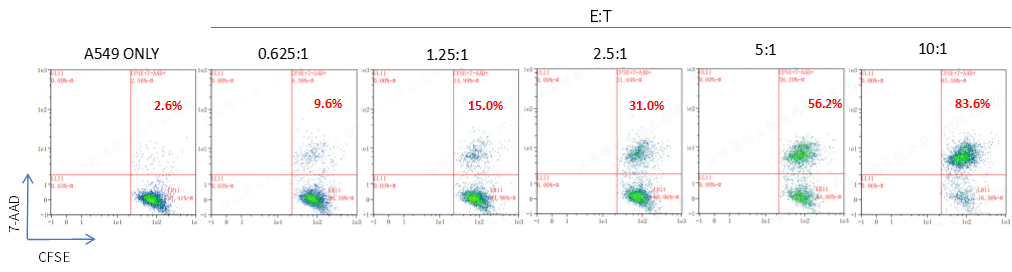
FACS analysis of the killing rate of NK cells against CFSE-labeled A549 target cells
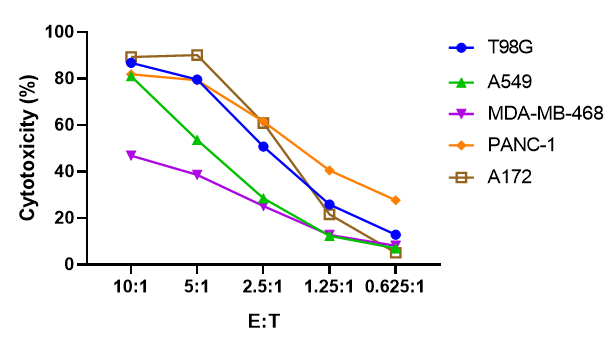
FACS analysis of the killing rate of NK cells against CFSE-labeled target cells of different solid tumors cells

Building 4, Yuewang Wisdom Valley, 1463 Wuzhong Avenue, Wuzhong District, Suzhou, China