Compared with the three-plasmid vector system, the four-plasmid vector system greatly reduces the possibility of RCL production and increases the safety of the vector system. The third generation lentiviral packaging platform contains four plasmids in the plasmid system, including a shuttle plasmid containing exogenous target genes and three helper plasmids which include two packaging plasmids and one envelope plasmid, respectively.
The premade plasmid products (HiPlas ™ LentiHelper Plasmids for T) on the platform plasmid system for lentiviral packaging of CAR-T, TCR-T and other lentivirus provided by Jiangsu Hillgene can meet the needs of customers at various stages of pre-clinical research, IIT, IND application, clinical trials and commercialization.
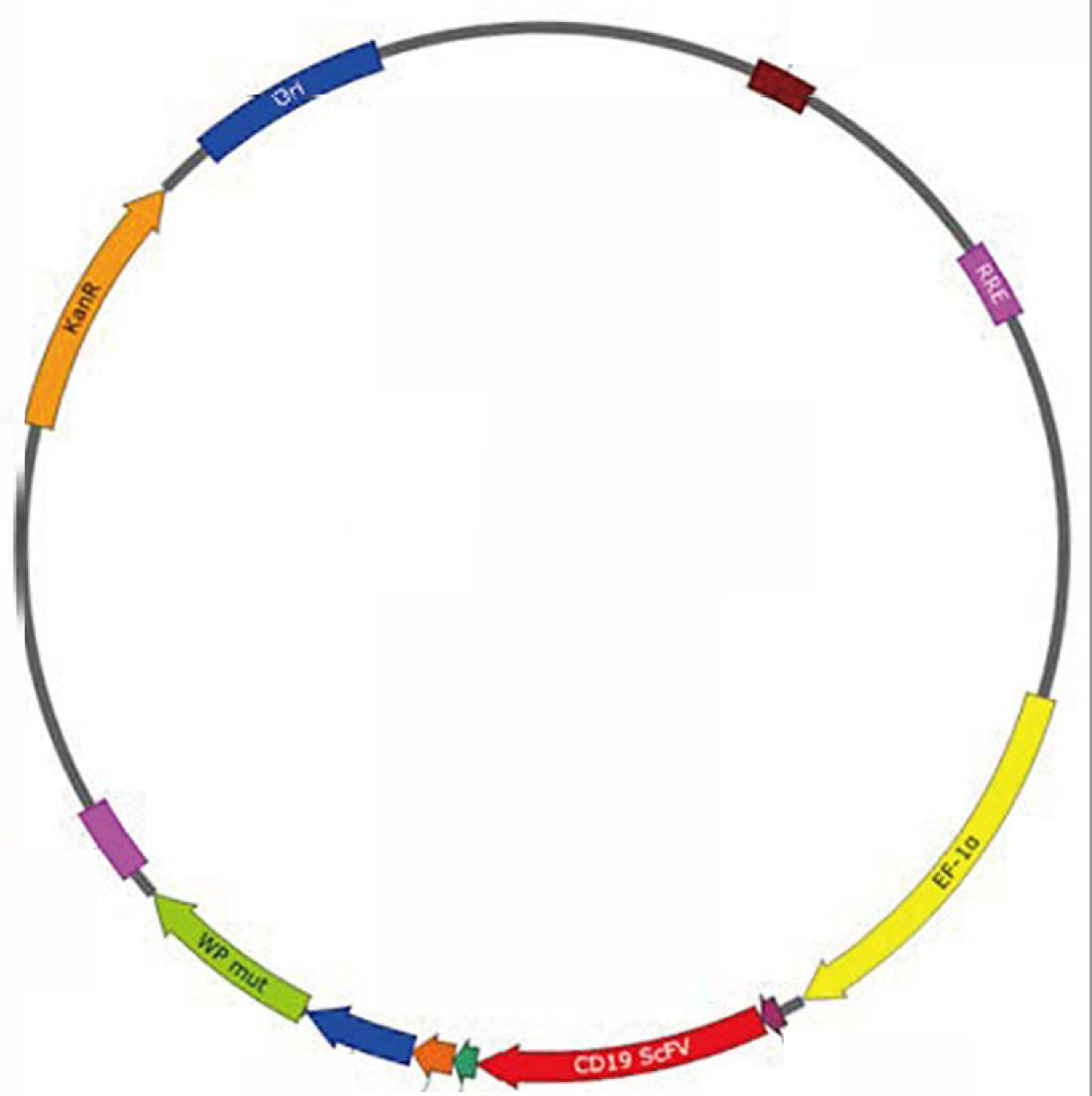
Premade Plasmid Advantages
The four-plasmid vector system independently developed by Jiangsu Hillgene meets the compliance and traceability of plasmid production, and efficiently supports lentiviral packaging, showing excellent infection efficiency at low MOI for T cells.·Premade plasmids: GMP-grade premade plasmids can meet the needs for different stages of a project;
·The four-plasmid vector system greatly reduces the possibility of RCL production and increases the safety of the vector system;
·The inclusion of modified WPRE elements can enhance the expression of target genes while taking into account safety (modified WPRE highlighted);
·The only compliant Kana resistance gene of marketed cell drugs was used for resistance;
·The optimization and modification of possibly carcinogenic sequences (the modification of wild-type WPRE elements to express the sequence of truncated X protein can avoid the risk of cancer);
·The optimization of promoter selection can take into account both efficiency and safety;
·After obtaining the DMF filing number, it can be directly cited in the IND application of FDA to provide detailed pharmaceutical study information;
·The IND application dossier of three helper plasmids has helped a number of cell drugs such as CAR-T and TCR-T obtain implied approval for clinical trials around the world.

Master File Acknowledgement Letter from FDA CBER
Premade Plasmid Particulars



Building 4, Yuewang Wisdom Valley, 1463 Wuzhong Avenue, Wuzhong District, Suzhou, China