A CD19 CAR-T vector (pHi-HV01-EF1α-CD19 CAR) with a specific killing function and convenient identification of infection efficiency has been developed by Jiangsu Hillgene based on the second-generation CAR-T technology. The vector is added with the costimulatory domain, 4-1BB, which prolongs the survival time of CAR-T cells and promotes the proliferation of cells, and the kanamycin resistance is also modified so that it can meet the requirements of domestic and foreign regulations and policies.
| Specification | 0.5ml/strip |
| Validity period | 24 months |
| Storage conditions | -80℃ |
| LOQ | NA |
| LOD | NA |
| IND Filing | NA |
| FDA Filing | NA |
CD19 CAR-T lentiviral packaging is performed using Jiangsu Hillgene’s VSV-G envelope four-plasmid system. VSV-G pseudotyped LVVs adhered to and entered the cell by recognizing the low-density lipoprotein receptor (LDL-R) on the cell surface. The prevalence of LDL-R on the cell membrane enabled VSV-G to allow LVVs to enter a variety of cells. At present, VSV-G pseudotyped LVVs are widely used in the transduction of human and other mammalian cells, and CD19 CAR-T can efficiently and specifically kill CD19-positive cells, which provides a very good research tool for preclinical studies of gene/cell therapy and is of great significance for promoting the development of gene/cell therapy.
CD19 CAR-T Vector Design

CD19 CAR-T Lentivirus Preparation Process

CD19 CAR-T Lentivirus Quality Standard
| Description | Detection items | Detection Method | Quality Standard | Result |
| DS | HCP | ELISA | Report the result | 3106.49ng/mL |
| DS/finished products | p24 | ELISA | Report the result | 13913.29/mL |
| TU | FACS(293T) | Report the result | 3.58E+08TU/mL | |
| Endotoxin | 2020 ChP1143 | <100EU/mL | Meet the requirement | |
| Mycoplasma | q-PCR | Shall meet the requirement | Negative | |
| Sterility | rapid test | Shall meet the requirement | Meet the requirement | |
| HCD | qPCR | Report the result | 64ng/mL |
*Specific products to be sold are determined according to the actual batch.
1. CD19 CAR-T Cell Preparation Process

2. CAR-T Detection Data
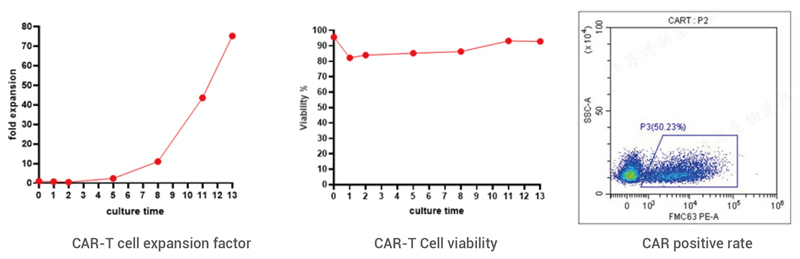
3. CAR-T Cell Quality Standard
| Detection items | Detection Method | Quality Standard | Result |
| CAR positive rate | FACS | ≥10.0% | 50.23% |
| CD3/4/8 | FACS | Report the result | 0.68 |
| Mycoplasma | qPCR | Shall meet the requirement | Negative |
| Sterility | Sterility rapid test | Shall meet the requirement | Negative |
| Product name | Cat.No. | specification |
| CD19 CAR-T Premade Lentivirus | HG-CT1901 | 0.5mL |