Product Name: Human Residual Total RNA Detection Kit (RT-PCR)
This kit is designed for the quantitative detection of residual Human total RNA in various biological products to improve control quality of nucleic acid.
This kit adopts the principle of the RT-PCR fluorescent probe, combining reverse transcription PCR.technology and fluorescent probe method, to realize one-step quantitative detection.
fg-level quantification (2–200,000 fg/μL)
One-step RT-qPCR – Faster workflow
Human-specific primers/probes – No cross-reactivity
Pre-mixed master mix – Reduce pipetting steps
18-month stability – -20°C storage
ROX options – Compatible with major qPCR instruments (ABI7500, CFX96, LightCycler)
Internal positive control (IPC) – Detects inhibition
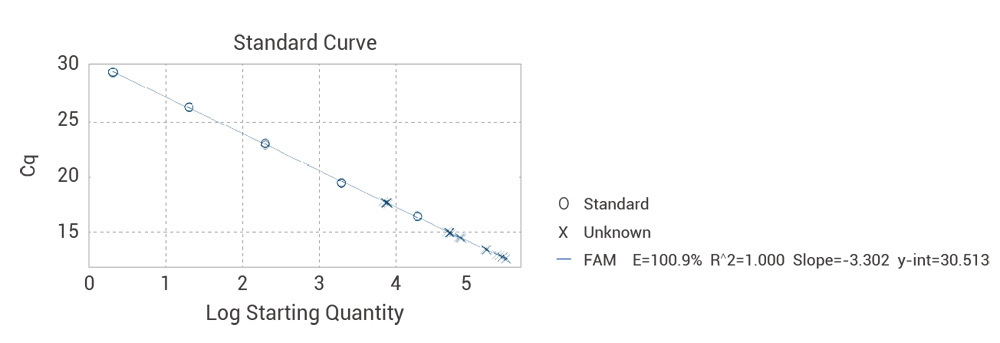
R²≥0.98 – Linear standard curve
DNase-treated RNA standards – Eliminate gDNA interference
1.Sample Preparation & Standard Dilution
2.Reaction Setup
3.PCR Amplification
4.Data Analysis
The Whole Process Takes About 2.5 Hours (including 1.5h for PCR run).
| Milestones | Specifications | Deliverables |
|---|---|---|
| Kit Preparation | - Pre-coated 96-well microplate with 293T HCP antibody - Ready-to-use TMB substrate and Stop Solution - Biotinylated detection antibody and HRP conjugate (requires dilution) - 293T HCP standard (81 μg/mL) | - Complete kit with all components - Detailed instructions for use |
| Assay Setup | - Double-antibody sandwich ELISA protocol - Detection range: 37–27,000 ng/mL - LOQ: 37 ng/mL - Precision: CV ≤10%, RE ≤±15% | - Optimized dilution scheme for standards - Pre-formulated wash buffers |
| Sample Processing | - Sample dilution in provided Diluent Buffer - pH adjustment to 6.0–8.0 if needed - Triplicate testing recommended | - Validated sample prep protocol - Negative control (PBS) included |
| Assay Execution | - Incubation: 1.5 h (RT) → Wash → Detection antibody (45 min) → HRP conjugate (30 min) → TMB (15 min) → Stop Solution | - Step-by-step workflow - Plate layout template |
| Data Analysis | - OD450 nm measurement - 4-parameter curve fitting for quantification - Example standard curve provided (R² ≥0.99) | - Raw and analyzed data - QC report (CV/RE validation) |
| QC & Validation | - Purity checks via SDS-PAGE/Western Blot (if tagged) - Spike recovery tests (2–10× sample concentration) - Inter-plate reproducibility checks | - QC certificates - Batch-specific performance data |
| Turnaround Time | - Standard protocol: 3–4 hours (excluding sample prep) - High-throughput: Customizable for 96/384-well formats | - Rush options available - Technical support included |
| Competitive Advantages | - Higher sensitivity: Lower LOQ (37 ng/mL) vs. market average (50 ng/mL) - Broad dynamic range: Covers 3 logs (37–27,000 ng/mL) - Ready-to-use: Minimal prep steps reduce user error | - Comparison table vs. competitors - Application notes (e.g., CHO/E. coli) |
Purpose: Detect trace human residual RNA (2fg/μL-200pg/μL) in biologics with superior sensitivity for complete nucleic acid safety assessment.
Feature:
One-step RT-qPCR technology combines reverse transcription and quantification in a single tube, reducing contamination risk
Dual probe system (FAM for target RNA/VIC for IPC) enables simultaneous inhibition monitoring
Application: Essential for cell/gene therapy products, vaccines, and recombinant biologics QC
Technical Effort:
Validated 6-point standard curve with R²≥0.98 and 85-110% amplification efficiency
Includes ROX calibrators for 12+ instrument platforms (ABI, Bio-Rad, Roche etc.)
Purpose: Simplify residual RNA testing with ready-to-use reagents and standardized protocols.
Feature: Pre-mixed master mix reduces hands-on time by 50% versus component assembly
18-month shelf life at -20°C ensures long-term reagent stability
Application: Ideal for lot release testing in biomanufacturing (CAR-T, monoclonal antibodies)
Technical Effort:
Includes IPC control with ≤1 Ct variation for inhibition detection
Validated with ≤15% inter-assay CV across triplicates
Purpose: Overcome PCR inhibition in protein-rich biologics samples.
Feature: Optimized enzyme mix maintains sensitivity in samples with up to 10mg/mL protein
DNase pretreatment protocol eliminates gDNA interference
Application: Critical for process development and final product release testing
Technical Effort:
Validated spike recovery (80-120%) across biologics matrices
Includes NTC control with ≤2fg/μL background signal
Purpose: Quantify residual host RNA in lentiviral vector batches to meet stringent purity requirements (<1pg/dose)
Feature:One-step RT-qPCR design simplifies workflow (15min setup)
Ultra-sensitive detection down to 2fg/μL (10x more sensitive than conventional kits)
Application:
Critical for lot-release testing of gene therapy vectors
Validated for both crude harvests and purified viral stocks
Technical Effort:
Cross-validated with digital PCR (R²=0.99)
Demonstrated ≤0.5Ct variation in triplicate wells
95-105% spike recovery across 20 production batches
Purpose: Monitor RNA clearance during CAR-T cell manufacturing
Feature:Built-in IPC controls identify PCR inhibition
ROX normalization ensures inter-instrument consistency
Application:
Optimized cell washing steps reduced residual RNA by 3 logs
Supports comparability studies for process changes
Technical Effort:
6-log dynamic range (2fg-200pg/μL)
Validated with 5 donor cell lines
18-month stability data for GMP use
Purpose: Detect host cell RNA in mRNA-LNP formulations
Feature:DNase-treated protocol eliminates DNA interference
Human-specific primers avoid plasmid false positives
Application:
Batch rejection for samples >10pg RNA/μg mRNA
Complies with FDA guidance on process residuals
Technical Effort:
100% concordance with NGS for >100nt fragments
≤15% CV across 3 operators
Compatible with ABI/BioRad/Roche platforms
| Specification | 100 Reactions |
Assay range | 2.00~2.00×104 fg/μL |
Limit of quantitation | 2.00 fg/μL |
Limit of detection | 0.50 fg/μL |
Precision | CV%≤15% |
| Validity period | 18 months |
| Storage conditions | -20℃ |
| LOQ | 2 fg/μL |
| LOD | 2 fg/μL |
| Concentration (fg/μl) | Log Concentration | Ct Value(1) | Ct Value(2) | Ct Value(3) | Ct Mean Value | Recovery rate |
| 2.00E+04 | 4.3 | 16.45 | 16.37 | 16.36 | 16.39 | 0.30% |
| 2.00E+03 | 3.3 | 19.41 | 19.51 | 19.44 | 19.46 | 0.26% |
| 2.00E+02 | 2.3 | 23.03 | 22.83 | 22.98 | 22.95 | 0.45% |
| 2.00E+01 | 1.3 | 26.31 | 26.33 | 26.26 | 26.3 | 0.12% |
| 2.00E+00 | 0.3 | 29.53 | 29.48 | 29.42 | 29.48 | 0.19% |
| Amplification efficiency | 100.90% | |||||
Standard curve: