 Regulation&Guideline(China)
Regulation&Guideline(China)NoRegulation&GuidelineIssued byRelease timeDownload01Technical Guideline for the Research of Human Cell Therapy and the Quality Control of Drug ProductsCDE2003/3/20doc02Con...
More+
Regulation&Guideline(China)
Regulation&Guideline(China)NoRegulation&GuidelineIssued byRelease timeDownload01Technical Guideline for the Research of Human Cell Therapy and the Quality Control of Drug ProductsCDE2003/3/20doc02Con...
More+
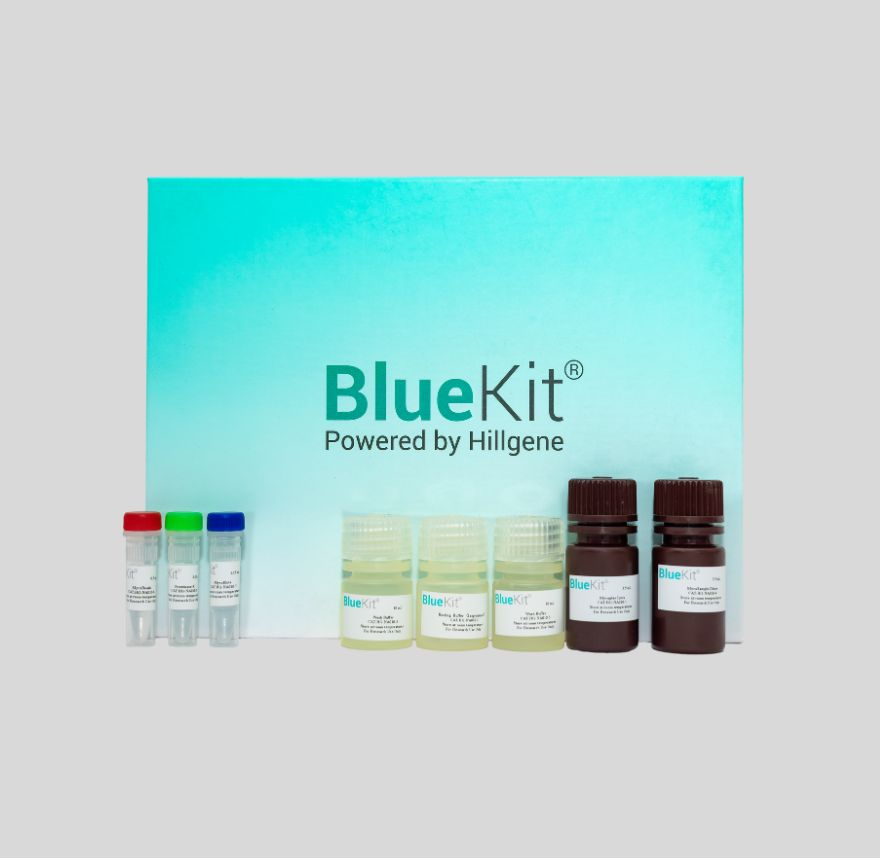
 Pichia Pastoris Hcp Elisa Assay Kit
This product uses a double antibody sandwich method to detect Pichia pastoris host protein residues in samples. Using a microtiter plate pre-coated with Pichia pastoris host protein capture antibody, ...
More+
Pichia Pastoris Hcp Elisa Assay Kit
This product uses a double antibody sandwich method to detect Pichia pastoris host protein residues in samples. Using a microtiter plate pre-coated with Pichia pastoris host protein capture antibody, ...
More+
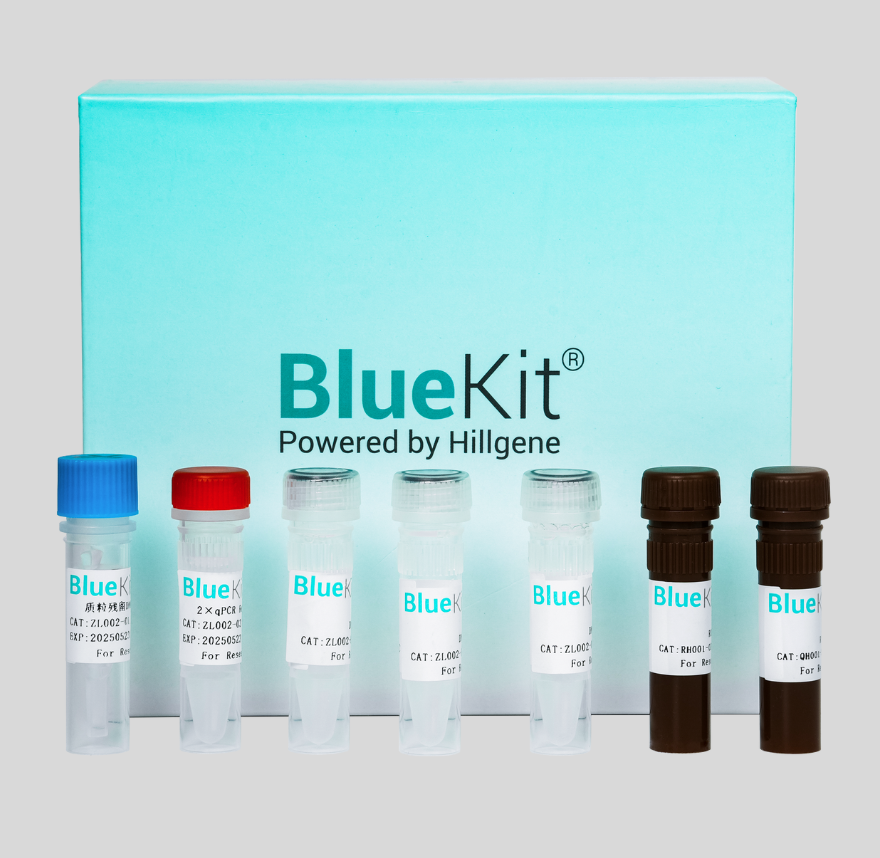
 Pichia Residual DNA Detection Kit (qPCR)
The Pichia pastoris expression system combines the advantages of both prokaryotic and eukaryotic expression systems. It offers several benefits, including low production costs, high-level protein expr...
More+
Pichia Residual DNA Detection Kit (qPCR)
The Pichia pastoris expression system combines the advantages of both prokaryotic and eukaryotic expression systems. It offers several benefits, including low production costs, high-level protein expr...
More+
 Company Profile
Hillgene, a leading biotech innovator, is headquartered in Suzhou's Wuzhong District near Taihu Lake, with a 10,000㎡ GMP-certified R&D and production base, complemented by manufacturing sites...
More+
Company Profile
Hillgene, a leading biotech innovator, is headquartered in Suzhou's Wuzhong District near Taihu Lake, with a 10,000㎡ GMP-certified R&D and production base, complemented by manufacturing sites...
More+
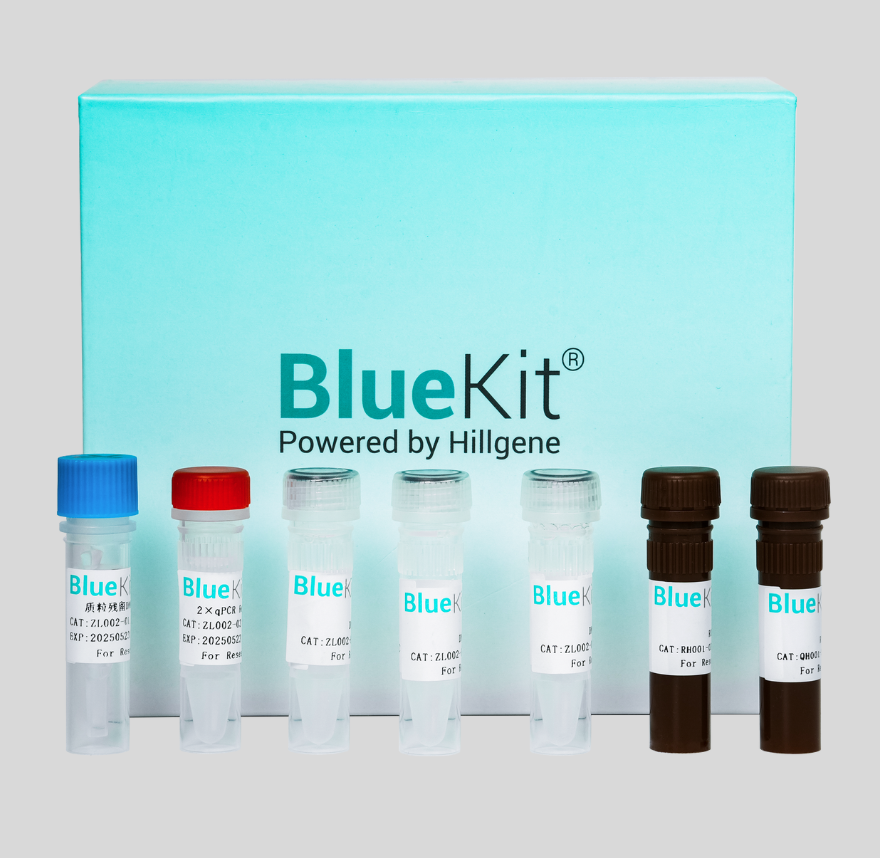 Host Cell DNA Residual Detection Kit for 293T(qPCR)
This kit is designed for the quantitative detection of the 293T cell DNA in intermediate, semi-finished and finished products of various biological products.This kit adopts the principle of the Taqman...
More+
Host Cell DNA Residual Detection Kit for 293T(qPCR)
This kit is designed for the quantitative detection of the 293T cell DNA in intermediate, semi-finished and finished products of various biological products.This kit adopts the principle of the Taqman...
More+
 CD-19 Lentivirus (VSV-G)
A CD19 CAR-T vector (pHi-HV01-EF1α-CD19 CAR) with a specific killing function and convenient identification of infection efficiency has been developed by Jiangsu Hillgene based on the second-generati...
More+
CD-19 Lentivirus (VSV-G)
A CD19 CAR-T vector (pHi-HV01-EF1α-CD19 CAR) with a specific killing function and convenient identification of infection efficiency has been developed by Jiangsu Hillgene based on the second-generati...
More+
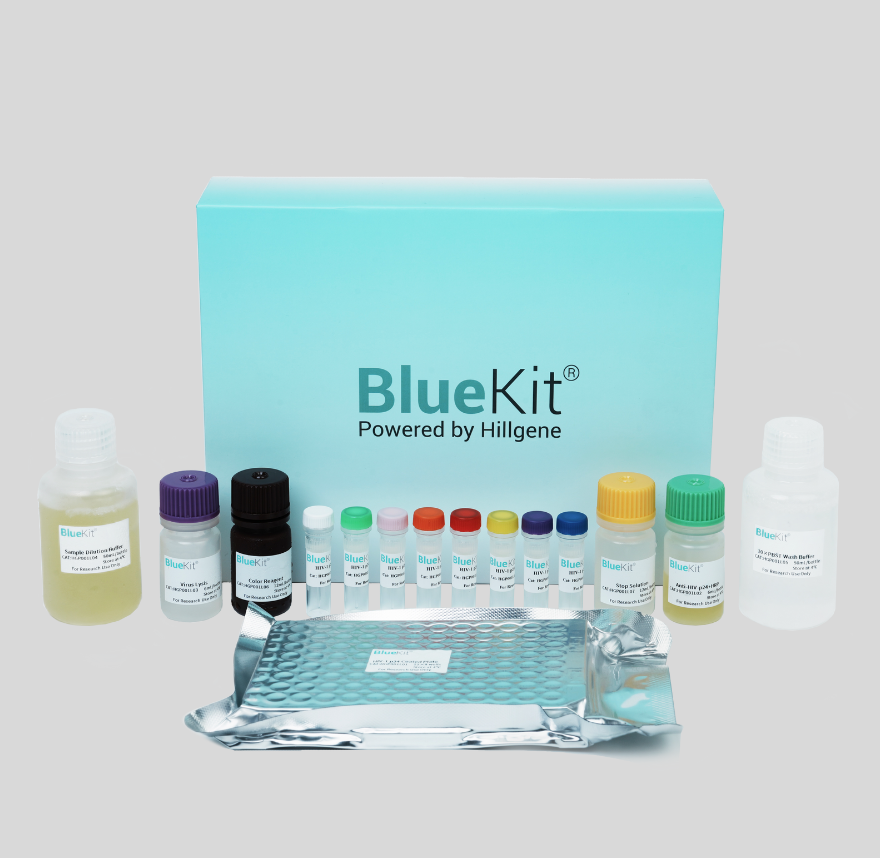
 HIV-1 p24 ELISA Detection Kit
This kit is designed for the quantitative detection of p24 protein content by using a double-antibody sandwich method, suitable to detect p24 protein content in any HIV-1 lentivirus product.
More+
HIV-1 p24 ELISA Detection Kit
This kit is designed for the quantitative detection of p24 protein content by using a double-antibody sandwich method, suitable to detect p24 protein content in any HIV-1 lentivirus product.
More+
 Another Award | Hillgene Was Awarded the TOP 5 Annual Pharmaceutical CXO Companies
From November 13 to 14, 2021, the 2021 Third Annual Bioprocess Industry Summit was successfully held in Shanghai. With the theme of "Latecomers Arrive Faster, Great Things Will Come", many e...
More+
Another Award | Hillgene Was Awarded the TOP 5 Annual Pharmaceutical CXO Companies
From November 13 to 14, 2021, the 2021 Third Annual Bioprocess Industry Summit was successfully held in Shanghai. With the theme of "Latecomers Arrive Faster, Great Things Will Come", many e...
More+
 Announcement of “Regulations on Manufacturing Quality Management for Chimeric Antigen Receptor T Cell (CAR-T cell)-based Drug Products”
In recent years, immunotherapy has experienced a series of rapid developments. New immunotherapy technologies represented by specific adoptive immune cell therapy and immune checkpoint antibody therap...
More+
Announcement of “Regulations on Manufacturing Quality Management for Chimeric Antigen Receptor T Cell (CAR-T cell)-based Drug Products”
In recent years, immunotherapy has experienced a series of rapid developments. New immunotherapy technologies represented by specific adoptive immune cell therapy and immune checkpoint antibody therap...
More+
 Regulation&Guideline(FDA)
Regulation&Guideline(FDA)No.Regulation&GuidelineIssued byRelease timeDownload01Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene TherapyFDA3/1998pdf02Eligibility Determination fo...
More+
Regulation&Guideline(FDA)
Regulation&Guideline(FDA)No.Regulation&GuidelineIssued byRelease timeDownload01Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene TherapyFDA3/1998pdf02Eligibility Determination fo...
More+
 Kanamycin ELISA Detection Kit
BlueKit® series Kanamycin ELISA Detection Kit is a specialized kit for quantitative detection of residual kanamycin content in drug substance, intermediates, and drug products of cell and gene thera...
More+
Kanamycin ELISA Detection Kit
BlueKit® series Kanamycin ELISA Detection Kit is a specialized kit for quantitative detection of residual kanamycin content in drug substance, intermediates, and drug products of cell and gene thera...
More+
 Host Cell DNA Fragment Residual Detection Kit for 293T(qPCR)
This kit is a kit specially designed for quantitative detection of the size distribution of residual 293T Cell DNA fragments in intermediates, bulk products and final products of various biological pr...
More+
Host Cell DNA Fragment Residual Detection Kit for 293T(qPCR)
This kit is a kit specially designed for quantitative detection of the size distribution of residual 293T Cell DNA fragments in intermediates, bulk products and final products of various biological pr...
More+
 Cell Residual Human IL-2 ELISA Detection Kit
BlueKit series cell residual human IL-2 ELISA detection kits use the double-antibody sandwich method to detect IL-2 protein in samples.
More+
Cell Residual Human IL-2 ELISA Detection Kit
BlueKit series cell residual human IL-2 ELISA detection kits use the double-antibody sandwich method to detect IL-2 protein in samples.
More+