![Hillgene Won the BioCon Awards [Annual CDMO Excellence Award] Hillgene Won the BioCon Awards [Annual CDMO Excellence Award]](/uploads/image/20250530/hillgene-won-the-biocon-awards-annual-cdmo-excellence-award.webp) Hillgene Won the BioCon Awards [Annual CDMO Excellence Award]
On September 6, BioCon Expo 2022, the 9th International Biopharmaceutical Conference and Exhibition, was held as scheduled at the Hangzhou International Expo Center. The BioCon Awards Ceremony and the...
More+
Hillgene Won the BioCon Awards [Annual CDMO Excellence Award]
On September 6, BioCon Expo 2022, the 9th International Biopharmaceutical Conference and Exhibition, was held as scheduled at the Hangzhou International Expo Center. The BioCon Awards Ceremony and the...
More+
 Newly Revised “Provisions for Drug Registration”
Chapter I General ProvisionsArticle 1 These Measures are formulated in accordance with the Drug Administration Law of the People's Republic of China (hereinafter referred to as the "Drug Admi...
More+
Newly Revised “Provisions for Drug Registration”
Chapter I General ProvisionsArticle 1 These Measures are formulated in accordance with the Drug Administration Law of the People's Republic of China (hereinafter referred to as the "Drug Admi...
More+
 Breaking | Hillgene Was Accepted as a Candidate of the “Unicorn” Enterprises Fostering Program
Science and technology are the primary productive forces. National and regional development cannot be separated from scientific and technological innovation. In order to implement the innovation-drive...
More+
Breaking | Hillgene Was Accepted as a Candidate of the “Unicorn” Enterprises Fostering Program
Science and technology are the primary productive forces. National and regional development cannot be separated from scientific and technological innovation. In order to implement the innovation-drive...
More+
 Notification of Public Comment Soliciting on “Technical Guideline for the Pharmaceutical Study and Evaluation of Cell-based Immunotherapy Products (Draft for Comments)”
In 2017, the former State Food and Drug Administration issued the "Technical Guidelines for Research and Evaluation of Cell Therapy Products (Trial)", which generally elaborated on the techn...
More+
Notification of Public Comment Soliciting on “Technical Guideline for the Pharmaceutical Study and Evaluation of Cell-based Immunotherapy Products (Draft for Comments)”
In 2017, the former State Food and Drug Administration issued the "Technical Guidelines for Research and Evaluation of Cell Therapy Products (Trial)", which generally elaborated on the techn...
More+
 Host Cell DNA Residual Detection Kit for PG13(qPCR)
The PG13 cell line was obtained by integrating a Moloney leukemia virus (MLV) expression vector and gibbon leukemia virus (GALV) membrane protein gene modification from mouse embryonic fibroblasts (NI...
More+
Host Cell DNA Residual Detection Kit for PG13(qPCR)
The PG13 cell line was obtained by integrating a Moloney leukemia virus (MLV) expression vector and gibbon leukemia virus (GALV) membrane protein gene modification from mouse embryonic fibroblasts (NI...
More+
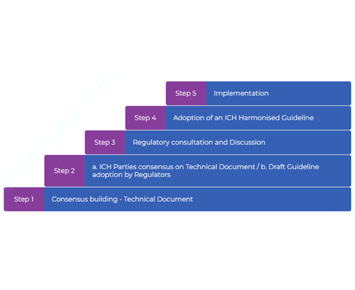 Congratulations!! Released Globally! ICH Guideline E19, with Academician Zhang Dan as a Working Expert, Was Formally Released and Reached Step 5
According to the ICH official website, ICH Guideline E19 has entered the Step 5 stage of full implementation by ICH members. Academician Zhang Dan, Vice Chairman of Spectrum Biopharma, participated in...
More+
Congratulations!! Released Globally! ICH Guideline E19, with Academician Zhang Dan as a Working Expert, Was Formally Released and Reached Step 5
According to the ICH official website, ICH Guideline E19 has entered the Step 5 stage of full implementation by ICH members. Academician Zhang Dan, Vice Chairman of Spectrum Biopharma, participated in...
More+
 Notification of Public Comment Soliciting on “Technical Guideline for the Pharmaceutical Study and Evaluation of Gene Therapy Products (Draft for Comments)”
Technical Guidelines for Pharmaceutical Research and Evaluation of Gene Therapy Products(Draft for Comments)ForewordIn recent years, with the continuous advancement of gene delivery systems and editin...
More+
Notification of Public Comment Soliciting on “Technical Guideline for the Pharmaceutical Study and Evaluation of Gene Therapy Products (Draft for Comments)”
Technical Guidelines for Pharmaceutical Research and Evaluation of Gene Therapy Products(Draft for Comments)ForewordIn recent years, with the continuous advancement of gene delivery systems and editin...
More+