According to the ICH official website, ICH Guideline E19 has entered the Step 5 stage of full implementation by ICH members. Academician Zhang Dan, Vice Chairman of Spectrum Biopharma, participated in the formulation of this international standard as one of the experts of the global ICH working group. In July 2017, the ICH Management Committee approved the formulation of E19, and in April 2019, a draft for comments was released, entitled "Optimization of Safety Data Collection".
Source: https://ich.org/page/efficacy-guidelines
In June of the same year, the Center for Drug Evaluation (CDE) of the National Medical Products Administration of my country released the original text, translation and public comment notice of the E19 draft. On September 27, 2022, the final version of the ICH guideline E19 came into effect. After feedback and revisions from experts from all walks of life around the world, the title of the final guideline was set as "A Selective Approach to Safety Data Collection in Specific Late-stage Pre-approval or Post-approval Clinical Trials".
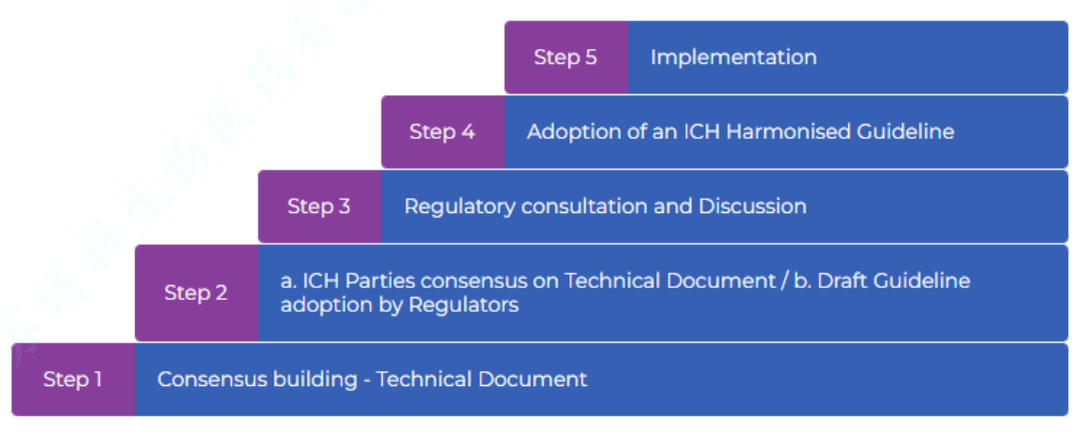
The five-step diagram of the formal ICH procedure (source: ICH official website)
The purpose of this guideline is to explain under what circumstances selective safety data collection methods are used. Safety and effectiveness are the gold standards for screening and elimination of new drug development, among which safety is the first. A reliable safety database provides an important basis for the safe use of new drugs in clinical trials. The guideline points out that during the development of new drugs and after the drug is launched, the sponsor collects a large amount of safety-related data, including all vital signs, laboratory data and adverse events, which are fully collected, collected, analyzed and evaluated. Under the condition of understanding the safety profile of the drug, in the later stages of drug development, as long as it can be guaranteed that it will not affect the patient's routine treatment, safety data can be selectively collected. The release of this guideline is of great significance for reducing the burden on patients and researchers and conducting clinical research more efficiently.

Source: https://www.cde.org.cn/ichWeb/guideIch/toGuideIch/3/0
Currently, the ICH Office of the Center for Drug Evaluation (CDE) of the National Medical Products Administration has not yet released the Chinese version of the ICH E19 guidelines. It is believed that the National Medical Products Administration (NMPA) will soon release the Chinese draft for comments on E19.
About Academician Zhang Dan
Academician Zhang Dan received a doctorate in medicine from Peking Union Medical College, a master's degree in public health from Harvard University, and a master's degree in healthcare management from the Wharton School of the University of Pennsylvania. Academician Zhang Dan has more than 20 years of experience in the pharmaceutical industry and was fully responsible for the clinical development and drug safety evaluation of the North American market at Sigma-Tau in Italy.
Academician Zhang Dan is currently a responsible expert for the National "Thirteenth Five-Year Plan" Major New Drug Creation Plan, and is involved in the formulation of technical guidelines, clinical review of new drugs, and training of drug review personnel at the Center for Drug Evaluation of the National Medical Products Administration. He is currently the leader of the International ICH E19 IFPMA Expert Committee and an expert of the NMPA ICH Working Group.
About ICH
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) is an international non-profit organization that has evolved since its founding in 1990. Since the announcement of its organizational reform in October 2015, ICH has grown to include 20 members and 35 observers. The mission of ICH is to achieve greater coordination worldwide to ensure that medicines are developed, registered and maintained in a resource-efficient manner so that they are safe, effective and of high quality while meeting high standards. In June 2017, the China National Medical Products Administration (NMPA) became a full member of ICH and was elected as a member of the ICH Management Committee in June 2018 to participate in the development of core international standards in the field of drug registration.
Specific document link: English draft for comments of E19