The kit is used to qualitatively detect the presence of mycoplasma contamination in master cell banks, working cell banks, virus seed lots, control cells, and cells for clinical therapy.
The kit uses qPCR-fluorescent probe technology to verify with reference to mycoplasma detectionrelated requirements in EP2.6.7 and JPXVII. It can cover more than 100 mycoplasmas and has no cross reaction with closely related strains. The detection is rapid which can be completed within 2 hours, with strong specificity.
50 Reactions.
| FAM(CT Value) | HEX(CT Value) | |||
| Negative | Positive | Negative | Positive | |
| 1 | NA | 32.65 | 33.26 | 32. .48 |
| 2 | NA | 34.26 | 32.29 | 33.23 |
| 3 | NA | 35.32 | 35.46 | 34.67 |
| 4 | NA | 31.51 | 34.79 | 31.68 |
| 5 | NA | 30.54 | 35.25 | 35.91 |
| 6 | NA | 34.97 | 33.57 | 34.2 |
| 7 | NA | 34.32 | 34.35 | 33.26 |
| 8 | NA | 38.45 | 35.87 | 35. .54 |
| 9 | NA | 33.67 | 33.24 | 33.12 |
| 10 | NA | 37.41 | 33.41 | 32.24 |
| Results of 10 mycoplasma standards | Results of 3 relevant bacteria | |||||
| Strain | Positive/Total | Strain | Positive/Total | L.acidophilus | S.pneumoniae | Clos. acetobutyleum |
| M.orale | 24/24 | M.synoviae | 24/24 | Negative | Negative | Negative |
| M.galliscepticum | 23/24 | M.arginini | 23/24 | |||
| A.laidlawii | 24/24 | M.hyorhinis | 24/24 | |||
| M.fermentans | 23/24 | Spiroplasma citri | 24/24 | |||
| M.pneumonia | 24/24 | M.saliuarium | 24/24 | |||
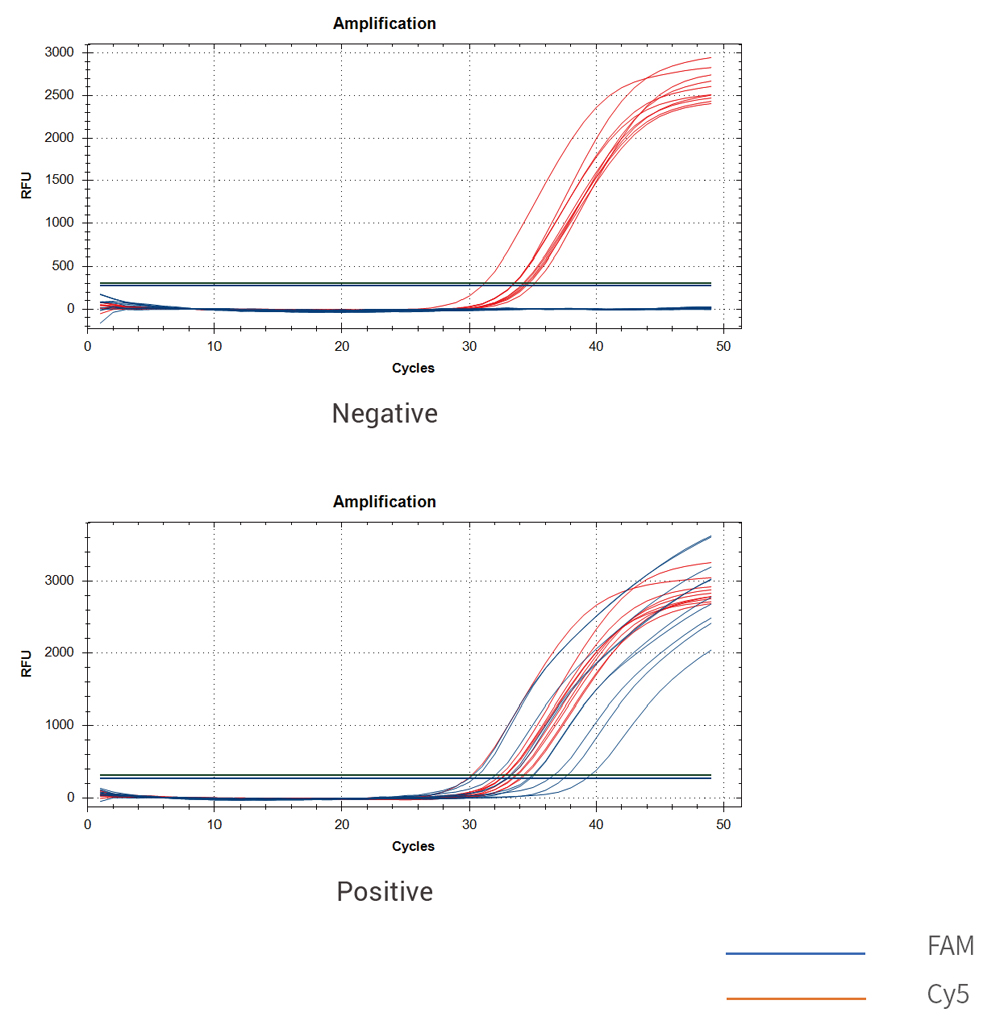
Test curve: