Product Name: Lentivirus Titer p24 ELISA Detection Kit
This lentivirus kit product uses a double-antibody sandwich method to detect HIV-1 p24 protein in samples. A monoclonal antibody specific to HIV-1 p24 antigen is coated on a microplate, and the standard or test sample is added into the reaction well. At the same time, the anti- HIV-1 p24 secondary antibody is added and incubated at room temperature to form the antibody-antigen-secondary antibody complex. The unconjugated compounds are removed by washing and protein content in the sample is indicated by the intensity of TMB color development.
Pre-fractionation of standards are ready-to-use for convenience and reliability.
Linear range from 1.37 - 1000 ng/mL, with as few as 1-2 dilution steps, reducing operational time.
Samples and enzyme-conjugated antibodies are incubated together, with only a single cleaning step, effectively saving detection time.
Clear standards, calibrated using NIBSC/WHO (90/636) standards, ensuring reliable results.
The kit has passed verification for specificity, accuracy, precision, linearity, quantitation limit, detection limit, durability, and stability in accordance with pharmacopeia standards. Production release is traceable, ensuring quality control.
Over 100 customers have used the product, with 10 IND submissions approved using it.
Optimization of the detection steps during product design minimizes detection bias caused by operator variability.
Short detection time, with results available in 1 hour, reducing systematic detection deviations.
Error proofing design: All components are color-coded to avoid confusion during use.
1.Sample Preparation& Sample Addition
2.Incubate Sample & EnzymeAntibody+ Cleaning
3.Developing&Termination
4.Reading
The Whole Processes Take About 1 Hours.
| Feature | Technical Highlights | Market Advantage |
|---|---|---|
| Universal Compatibility | • Compatible with diverse host cells (CHO, E. coli, Vero, Human, plasmids, SV40LTA & EIA). • Works with varied sample types (liquid, dry powder) and pH ranges (6.0–8.0). | Outperforms kits limited to specific matrices or requiring rigid pH adjustments. |
| High-Sensitivity DNA Recovery | • Glycogen and yeast tRNA enhance trace DNA capture (detection limit: <1 pg/μL). • Includes spiked controls (2–10× sample DNA) and triplicate processing for precision. | Superior recovery rates vs. silica-column methods, especially for low-abundance DNA in biologics. |
| Rapid & Automated Workflow | • Magnetic bead-based protocol (≤1.5 hours). • Pre-optimized buffers (lysis/wash/elution) reduce hands-on steps. • Compatible with high-throughput qPCR workflows. | 50% faster than phenol-chloroform extraction, with minimal manual intervention. |
Purpose: Accurately measure lentiviral physical titer (1.37–1000 ng/mL p24) to ensure batch consistency and transduction efficiency.
Feature:Double-antibody sandwich ELISA with anti-HIV-1 p24 capture + HRP-conjugated detection antibodies for high specificity
Pre-coated plates reduce hands-on time; <5% CV between replicates for reliable data
Application: Critical for lentiviral vector production (e.g., CAR-T/NK therapies, gene editing) and process optimization
Technical Effort:
Validated with 7-point standard curve (R² > 0.98) and internal controls (70–130% recovery)
Compatible with cell supernatants, purified virus, or lysed samples (viral lysis buffer included)
Purpose: Enable rapid, scalable titer analysis with minimal steps
Feature:1-hour total protocol (30-min incubation + 10-min development) vs. traditional 3–4 hr kitsReady-to-use reagents (pre-diluted standards, single-wash buffer) reduce prep errors
Application: Ideal for GMP/GLP-compliant labs requiring fast turnaround (e.g., lot release testing)
Technical Effort:
Includes extraction recovery control (ERC) to validate matrix effects (e.g., serum, media)
Optimized for automated plate readers (450 nm/630 nm dual-wavelength detection
Purpose: Overcome interference from complex samples (e.g., serum, cell debris)
Feature:Virus lysis buffer ensures complete p24 release without false negatives
Sample Diluent Buffer minimizes background noise for low-titer samples (≥1.37 ng/mL)
Application: Suitable for upstream/downstream process monitoring in viral vector manufacturing
Technical Effort:
Validated spike-in recovery (20–200 ng/mL) with 85–115% accuracy
Pre-optimized wash steps (4× PBST) eliminate non-specific binding
Purpose: Rapid quantification of lentiviral physical titer (p24) to ensure consistent viral vector production for CAR-T cell therapy.
Feature:96-well format enables parallel testing of 80+ samples in under 1.5 hours, accelerating batch release.
Broad detection range (1.37–1000 ng/mL) covers diverse lentiviral prep concentrations without repeat dilution.
Application:
Critical for GMP-compliant lentiviral vector manufacturing, with less than 20% CV between replicates.
Used by Hillgene’s CD19 CAR-T team to validate 10^8–10^10 VP/mL batches (data on file).
Technical Effort:
Validated against qPCR (R²=0.98) and legacy ELISA kits (90–110% recovery).
Pre-coated plates reduce hands-on time compared to traditional sandwich ELISA.
Purpose: Resolve AAV-LV cross-contamination risks by specific p24 detection in mixed viral preps.
Feature:HIV-1 p24 monoclonal antibodies show zero cross-reactivity with AAV capsid proteins (VP1/2/3).
1.37 ng/mL sensitivity detects low-abundance LV in AAV-dominant samples.
Application:
Critical for dual-vector gene therapy projects requiring AAV:LV ratio verification.
Technical Effort:
Spike-recovery tests in AAV8 samples achieved 92–108% accuracy.
Compared favorably to commercial kits (Merck, Takara) with 30% faster protocol.
Purpose: Eliminate titer variability in lentivirus packaging by monitoring p24 during upstream production.
Feature:Integrated lysis buffer eliminates pre-processing steps—directly load crude supernatants or purified virus.
Internal control (200 ng/mL) ensures system suitability (70–130% recovery).
Application:
Identified 3X titer improvement in Hillgene’s 293T packaging system by correlating p24 levels with transfection parameters.
Technical Effort:
ERC recovery validation (85–115%) confirms minimal matrix interference from cell debris/media components.
Linear standard curve (R²>0.99) across 5 independent runs.
| Specification | 96 Test |
Assay range | 1.37-1000ng/mL |
Sensitivity | 0.35ng/mL |
Precision | CV%≤10%, RE%≤±15% |
| Validity period | 12 months |
| Storage conditions | 4℃ |
| LOQ | 1.37ng/mL |
| LOD | 1 ng/mL |
Lentivirus Titer p24 Rapid ELISA Detection Kit is used for p24 protein and titer detection of HIV-1 lentiviral samples. It is rapid (pre-fractionation of standards), convenient (single incubation and single wash), and stable. Experimenter can be used to complete the detection of the p24 protein of HIV-1 lentiviral samples in less than one hour.
| Batch No. | Hillgene(ng/mL) | Brand T (ng/mL) | CV |
| A | 6.58E+03 | 5.19E+03 | 17% |
| B | 4.88E+03 | 3.35E+03 | 26% |
| C | 5.15E+03 | 3.42E+03 | 29% |
| D | 5.53E+03 | 4.94E+03 | 8% |
| E | 3.85E+03 | 3.33E+03 | 10% |
| F | 3.77E+03 | 3.26E+03 | 10% |
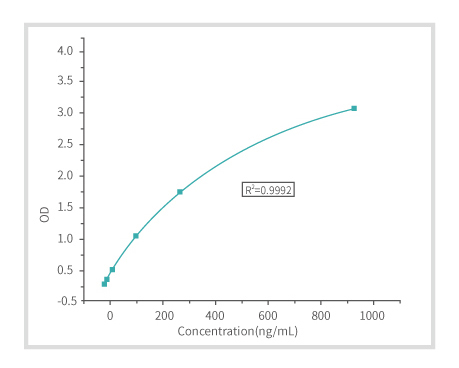
Figure 1: Standard Curve (Four-ParameterFit)
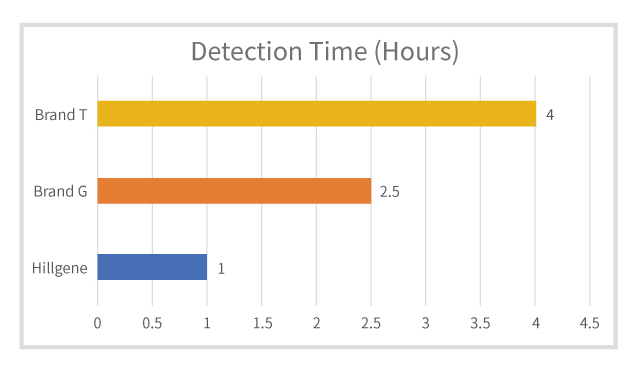
Figure 2: Comparison of Detection Times with Competitor p24 ELISA Products
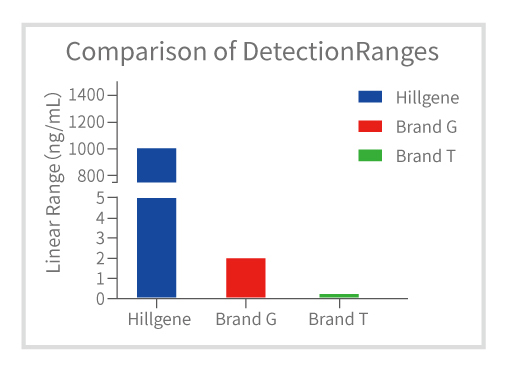
Figure 3: Comparison of Detection Range with Competitor p24 Products
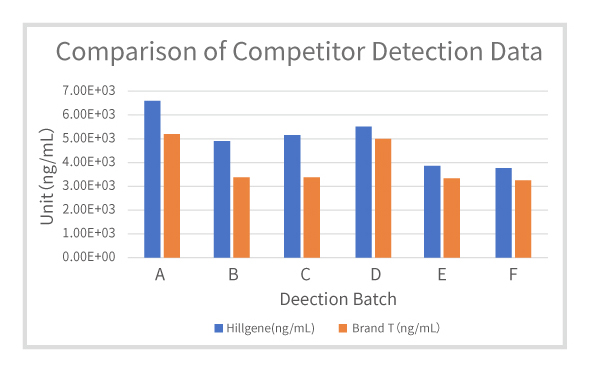
Figure 4: Comparison of Competitor Detection Data