Product Name: Human Residual DNA Fragment Analysis Detection Kit (qPCR)
This kit is designed for the quantitative detection of the size distribution of Human residual host cell DNA fragments in intermediates, semi-finished and finished products of various biological products.
This kit adopts the principle of PCR fluorescent probe method to quantitatively detect the size distribution of human residual host cell DNA fragments in the sample. The kit features three different amplified fragments (99 bp, 200 bp and 307 bp), and the Human DNA quantification reference is used to make standard curves for different amplified fragments respectively, and the fragment distribution of Human residual DNA in the sample is analyzed through the ratio of different sizes of fragments.
The kit is a rapid, specific and reliable device, with the minimum detection limit reaching fg level.
fg-level quantification (2–200,000 fg/μL)
One-step RT-qPCR – Faster workflow
Human-specific primers/probes – No cross-reactivity
Pre-mixed master mix – Reduce pipetting steps
18-month stability – -20°C storage
ROX options – Compatible with major qPCR instruments (ABI7500, CFX96, LightCycler)
IPC included – Inhibitor detection
R²≥0.98 – Linear standard curves
50-150% recovery rate – Validated performance
1.Sample Preparation & Standard Dilution
2.Plate Setup & Loading
3.qPCR Amplification
4.Data Analysis
The Whole Process Takes About 3 Hours (including 2h for qPCR run).
| Feature | Technical Highlights | Market Advantage |
|---|---|---|
| Multi-Fragment Size Analysis | • Simultaneously detects 99bp, 200bp and 307bp DNA fragments • Quantitative analysis of fragment size distribution | Unique capability to characterize DNA degradation patterns, unlike standard single-fragment detection kits |
| Ultra-Sensitive Quantification | • Detection range: 3×10¹-3×10⁵ fg/μL (fg-level sensitivity) • Validated for compliance with pharmacopeial standards | 100x more sensitive than conventional gel electrophoresis methods for residual DNA analysis |
| Streamlined qPCR Workflow | • Pre-optimized primer/probe mixes for all three fragments • Includes IPC control and ROX reference dyes • Compatible with major qPCR platforms (ABI 7500, Bio-Rad CFX96, etc.) | Reduces setup time by 50% compared to designing and validating multiple fragment assays separately |
Purpose: Accurately quantify and analyze residual human DNA fragment distribution (99bp, 200bp, 307bp) in biologics for safety assessment.
Feature:
Triple-amplicon design (99/200/307bp) enables complete fragment distribution analysis in one test
TaqMan probe technology ensures 100x higher sensitivity than gel electrophoresis methods
Application: Critical for cell/gene therapy products, vaccines, and recombinant protein QC
Technical Effort:
Validated 5-point standard curve (3×10¹-3×10⁵ fg/μL) with R²≥0.98
Includes IPC control for PCR inhibition monitoring
Purpose: Simplify fragment analysis with ready-to-use reagents and standardized protocols.
Feature:
Pre-mixed master mixes reduce hands-on time by 60% versus component assembly
Dual ROX references ensure compatibility across 12+ qPCR platforms
Application: Ideal for lot release testing in biomanufacturing (CAR-T, mAbs)
Technical Effort:
Validated spike recovery (50-150%) with ≤1.0 Ct triplicate variation
18-month shelf life at -18°C
Purpose: Overcome matrix effects in protein-rich biologics samples.
Feature:
Optimized buffer system maintains sensitivity in high-protein samples (up to 10mg/mL)
Size-specific quantification meets FDA/EMA guidelines for residual DNA risk assessment
Application: Essential for process development and product characterization
Technical Effort:
Includes NTC/NCS controls for contamination monitoring
Validated with ≤15% inter-assay CV
Purpose: To accurately quantify and characterize residual host DNA fragments in CAR-T cell therapy products
Feature:Simultaneous detection of three fragment sizes (99bp, 200bp, 307bp) in single reaction
Broad dynamic range (3×10¹-3×10⁵ fg/μL) covers complete purification process monitoring
Application:
Critical quality control for IND-enabling studies of cell therapies
Validated for both viral vector and cellular therapy products
Technical Effort:
Demonstrated ≤1.0 Ct variation across triplicate wells
Achieved R²>0.98 for all three fragment standard curves
85-110% amplification efficiency across all targets
Purpose: To optimize purification processes by monitoring DNA fragment clearance
Feature:Fragment size distribution analysis identifies process bottlenecks
IPC Mix included for reaction quality control
Application:
Enabled 3X improvement in DNA clearance during AAV purification process development
Supports comparison of different nuclease treatment protocols
Technical Effort:
Validated against digital PCR (concordance >95%)
Demonstrated 50-150% spike recovery across all fragment sizes
Compatible with major qPCR platforms (ABI, BioRad, Roche)
Purpose: To demonstrate process comparability for regulatory submissions
Feature:Quantitative fragment profiling meets ICH Q5A requirements
ROX normalization ensures inter-instrument consistency
Application:
Accepted in multiple EMA/FDA filings for biosimilar approval
Supports manufacturing scale-up and process changes
Technical Effort:
Cross-validated with capillary electrophoresis
18-month stability data supporting GMP use
≤10% inter-lab variability in multicenter study
| Specification | 300 Reactions |
Assay range | 3.00×101~3.00×105 fg/μL |
Limit of quantitation | 3.00×101 fg/μL |
Precision | CV%≤15% |
| Validity period | 18 months |
| Storage conditions | -20℃ |
| LOQ | 30 fg/μL |
| LOD | 30 fg/μL |
| IND Filing | OK |
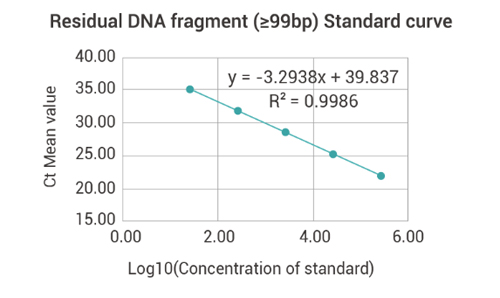
Residual DNA Fragment(≥99bp)Detection
| Standard | Ct Value | Ct-IPC value | |||
| Concentration (fg/μl) | Log10 (Concentration) | Ct Value | Mean value | Ct-IPC value | Mean value |
| 3.00E+05 | 5.48 | 21.66 | 21.66 | 22.96 | 22.8 |
| 3.00E+04 | 4.48 | 25.06 | 25.06 | 22.67 | |
| 3.00E+03 | 3.48 | 28.61 | 28.61 | 22.81 | |
| 3.00E+02 | 2.48 | 31.85 | 31.85 | 22.7 | |
| 3.00E+01 | 1.48 | 34.74 | 34.74 | 22.86 | |
| Amplification efficiency | 101.19% | ||||
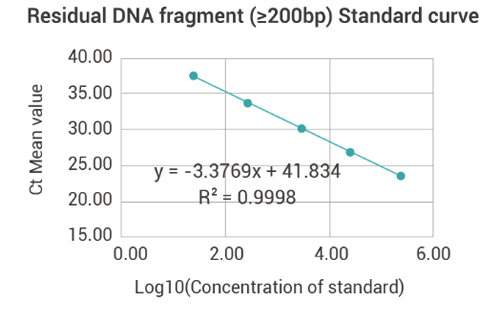
Residual DNA Fragment(≥200bp)Detection
| Standard | Ct Value | Ct-IPC value | |||
| Concentration | Log10 | Ct Value | Mean value | Ct-IPC value | Mean value |
| (fg/μl) | (Concentration) | ||||
| 3.00E+05 | 5.48 | 23.23 | 23.23 | 23.09 | 23.06 |
| 3.00E+04 | 4.48 | 26.8 | 26.8 | 23.08 | |
| 3.00E+03 | 3.48 | 30.16 | 30.16 | 22.99 | |
| 3.00E+02 | 2.48 | 33.49 | 33.49 | 23.13 | |
| 3.00E+01 | 1.48 | 36.77 | 36.77 | 23 | |
| Amplification efficiency | 97.76% | ||||
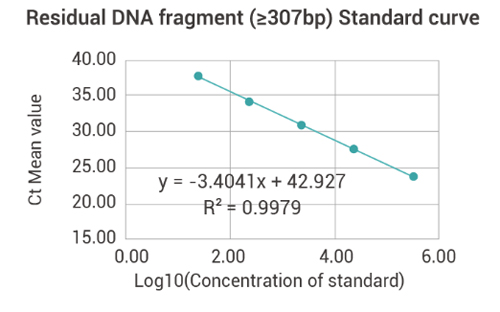
Residual DNA Fragment(≥307bp)Detection
| Standard | Ct Value | Ct-IPC value | |||
| Concentration | Log10 | Ct Value | Mean value | Ct-IPC value | Mean value |
| (fg/μl) | (Concentration) | ||||
| 3.00E+05 | 5.48 | 23.98 | 23.98 | 23 | 22.99 |
| 3.00E+04 | 4.48 | 27.98 | 27.98 | 22.99 | |
| 3.00E+03 | 3.48 | 31.31 | 31.31 | 23.04 | |
| 3.00E+02 | 2.48 | 34.4 | 34.4 | 22.88 | |
| 3.00E+01 | 1.48 | 37.79 | 37.79 | 23.02 | |
| Amplification efficiency | 96.68% | ||||