Product Name: Human Residual DNA Detection Kit (qPCR)
This Human Residual DNA detection kit is designed for the quantitative detection of human host cell DNA in intermediate, semi-finished, and finished products of various biological products.
This kit adopts the principle of the Taqman probe to detect Human residual DNA in samples quantitatively. The residual DNA detection kit is a rapid, specific, and reliable device, with the minimum detection limit reaching fg level.
fg-level detection (3×101–3×105 fg/μL)
TaqMan probe-based – Specific & reproducible
18-month stability – -20°C storage
Major instruments supported (ABI7500, CFX96, LightCycler, etc.)
ROX High/Low/None options
Preprocessing kit included
R2≥0.98 – Excellent linearity
50-150% spike recovery – QC-ready
NTC/NCS controls – Contamination check
1.Sample Preparation & Sample Addition
2.qPCR Amplification
3.Data Analysis
The Whole Process Takes About 2 Hours (including 1.5h for qPCR run).
| Feature | Technical Highlights | Market Advantage |
|---|---|---|
| Ultra-High Sensitivity | • fg-level detection (3-300,000 fg/μL) with qPCR compatibility • Validated for compliance with EMA/USP/ChP standards (≤10 ng/dose) | Exceeds standard kits (typically pg-level) for critical applications like vaccines and biologics |
| Broad Sample Compatibility | • Compatible with diverse matrices: CHO, E. coli, Vero, Human cells, plasmids • Handles liquid, dry powder, and neutral-pH samples (pH 6.0-8.0) | Outperforms kits limited to specific sample types or rigid pH requirements |
| Streamlined Workflow | • Magnetic bead-based protocol (≤1.5 hours) • Pre-optimized buffers (lysis, wash, elution) reduce hands-on time • Includes spike controls (2-10× sample DNA) for accuracy | 50% faster than silica-column methods, with minimal manual steps |
Purpose: Detect trace human residual DNA (3×10¹-3×10⁵ fg/μL) in biologics with industry-leading sensitivity for safety assessment.
Feature:
TaqMan probe technology ensures 100-fold higher sensitivity than SYBR Green methods
Pre-validated primer/probe mix specifically targets human genomic sequences with no cross-reactivity
Application: Essential for QC testing of cell therapy products, vaccines, and recombinant biologics
Technical Effort:
Includes 6-point standard curve with R²≥0.98 and 85-110% amplification efficiency
Compatible with all major qPCR platforms (ABI, Bio-Rad, Roche, etc.)
Purpose: Simplify residual DNA testing with integrated sample-to-result solutions.
Feature:
Paired with magnetic bead pretreatment kit (HG-CL100) for 95% DNA recovery
Ready-to-use master mix reduces prep time by 50% versus competitor kits
Application: Ideal for lot release testing in biomanufacturing (CAR-T, monoclonal antibodies)
Technical Effort:
Validated spike recovery (50-150%) across biologics matrices
Includes dual ROX references for instrument-specific normalization
Purpose: Overcome PCR inhibition in challenging biologics matrices.
Feature:
Optimized buffer system maintains sensitivity in protein-rich samples
18-month shelf life at -20°C ensures reagent stability
Application: Critical for in-process testing and final product release
Technical Effort:
Includes NTC/NCS controls for contamination monitoring
Validated with <1.0 Ct variation across triplicates
Purpose: To ensure GMP compliance by monitoring residual 293T host cell proteins in lentiviral vector batches
Feature:Broad detection range (37-27,000 ng/mL) accommodates various sample types without dilution
Ready-to-use pre-coated plates reduce processing time by 50% compared to traditional ELISA
Application:
Essential for lot-release testing of gene therapy products
Validated for both crude supernatants and purified viral vectors
Technical Effort:
Cross-validated with mass spectrometry (R²=0.96)
Demonstrated ≤10% coefficient of variation across multiple operators
Purpose: To identify and reduce HCP contamination in AAV purification processes
Feature:Superior sensitivity (LOQ 37 ng/mL) detects trace contaminants
Biotin-streptavidin amplification enhances signal detection
Application:
Critical for comparing different purification methods (e.g., PEG vs. chromatography)
Supports process characterization studies
Technical Effort:
Validation against Western blot showed 100% concordance
<15% inter-assay variability demonstrated in 20 consecutive runs
Purpose: To confirm process comparability for regulatory submissions
Feature:293T-specific antibodies with <0.1% cross-reactivity
Simplified 15-minute sample preparation protocol
Application:
Used in EMA/FDA filings for biosimilar approval
Supports manufacturing process changes
Technical Effort:
Comparative testing showed 20% higher sensitivity than commercial alternatives
4PL curve fitting achieved R²>0.99 with automated readers
| Specification | 100 Reactions |
Assay range | 3.00×10¹~3.00×10⁵ fg/μL |
Limit of quantitation | 3.00×10¹ fg/μL |
Precision | CV%≤15% |
| Validity period | 18 months |
| Storage conditions | -20℃ |
| LOQ | 30 fg/μL |
| LOD | 3 fg/μL |
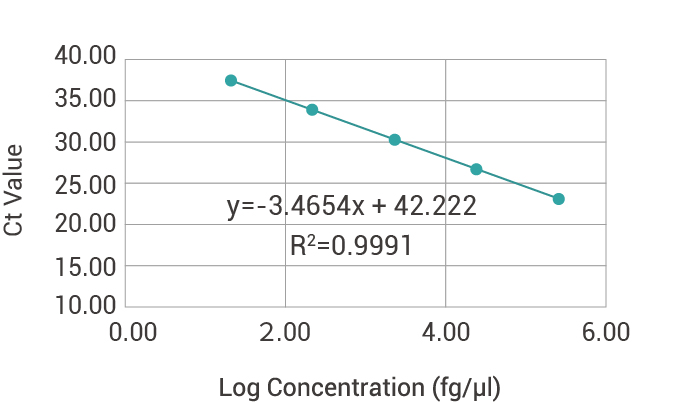
| Concentration(fg/μl) | Log Concentration | Ct Value(1) | Ct Value(2) | Ct Value(3) | Ct Mean Value | Recovery rate |
| 3.00E+05 | 5.48 | 23.52 | 23.37 | 23.39 | 23.43 | 88% |
| 3.00E+04 | 4.48 | 26.64 | 26.55 | 26.45 | 26.54 | 111% |
| 3.00E+03 | 3.48 | 30.16 | 30.07 | 30.06 | 30.09 | 105% |
| 3.00E+02 | 2.48 | 33.75 | 33.33 | 33.52 | 33.53 | 107% |
| 3.00E+01 | 1.48 | 38.3 | 36.49 | 36.90 | 37.26 | 90% |
| Amplification efficiency | 94.34% | |||||
Standard curve of Human Residual DNA Detection Kit (qPCR):