BlueKit®, a brand under Hillgene, offers genetically Modified K562 feeder cell product that express multiple cytokines including IL-21. When combined with mainstream immune cell culture media, NK cells derived from cord blood and PBMC, or tumour-infiltrating TIL cells, can be directionally activated and massively expanded in vitro under the synergistic effects of multiple cytokine signals, with the resulting NK and TIL cells exhibiting high purity. Currently, several NK cells and TILs cell products using feeder cells have been approved for clinical use or marketed globally, and studies have shown that genetically modified K562 feeder cells can achieve more than 10,000-fold expansion of NK or CAR-NK cells in 15 days, which can meet the demand for their large-volume preparation.

Pre-irradiated, multi-cytokine enhanced for superior NK/TIL expansion.
Validated for both cord blood and PBMC sources - CAR-ready.
Pre-qualified for IND submissions with full characterization data.
D0: Plasma and PBMC isolation, inoculation and activation
D4: Observe and medium exchange
D6: Observe and medium infusion
D8: Observe and medium infusion, re-activation
D10: Observe and medium exchange
D12: Observe and medium infusion
D14: Harvest
| Performance Program | Indicator results |
| NK/CAR-NK cell expansion | Over 5000 times (14d) |
| NK/CAR-NK cell viability | > 90% |
| NK/CAR-NK Cell purity | > 95% |
Fold expansion and viability of different Donor-derived NK cells:

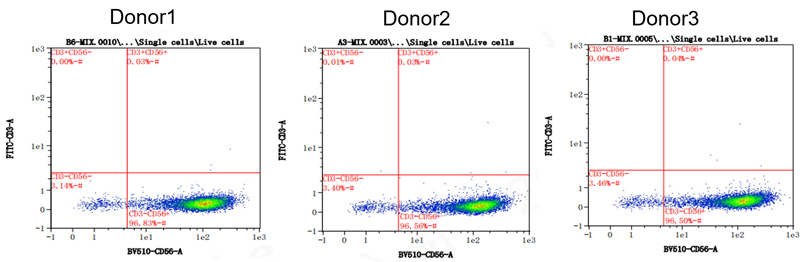
Purity of different Donor-derived NK cells:
Overview of Safety Endpoints | |
Radiation condition | X-ray, 100 Gy |
Cell proliferation | No proliferation |
Sterility test | Negative |
Endotoxin level | 2 EU/mL |
Mycoplasma | Negative |
RCL | Negative |
Cell transformation | Negative |
Cell tumorigenicity | Negative |
Residual K562 cells in NK cells | < 0.1% |