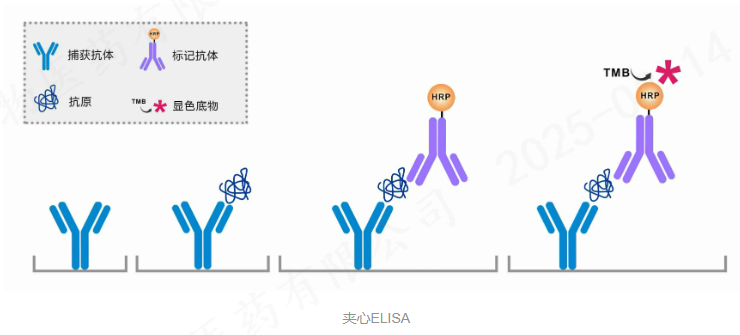
This kit is designed for the quantitative detection of HCP (host cell protein) content in biopharmaceuticals expressed on E.coli by using a double-antibody sandwich method.
This kit can be used to detect all components of HCP (host cell protein) in E.coli.
Reliable Performance: Stable and reliable standard curve with ~100% recovery across all concentrations.
Rapid Analysis: Total assay time requires only 2.5 hours.
Ultra-High Sensitivity: Detection limit as low as 0.3 ng/mL for precise trace-level analysis.
HCP residue control in recombinant protein production (e.g., insulin, cytokines) and plasmid DNA manufacturing.
Host impurity detection for viral vectors, mRNA vaccines, and related products.
Purity validation of plasmid-based raw materials.
| Specification | 96 Test |
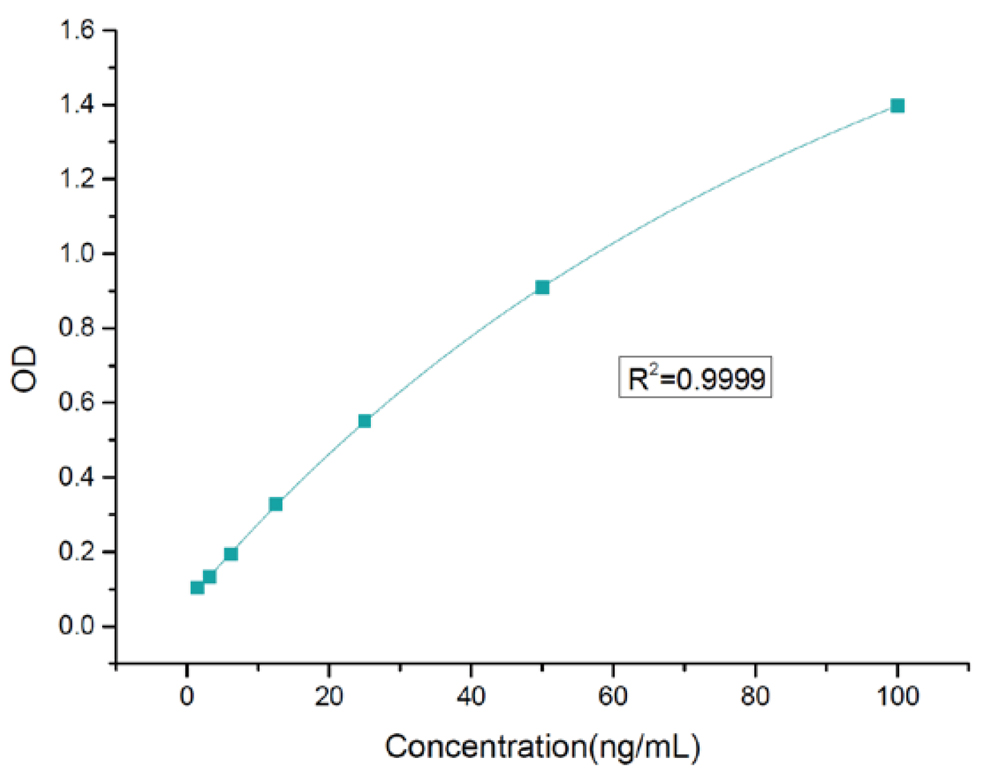
Detection range | 1.5625 - 100 ng/mL |
Limit of quantitation | 1 ng/mL |
Limit of detection | 0.3 ng/mL |
Precision | CV% ≤ 10%, RE% ≤ ±15% |
| Validity period | 12 months |
| Storage conditions | -20℃ |
| LOQ | 3 ng/mL |
| LOD | 0.3 ng/mL |
| IND Filing | |
| FDA Filing |
.png)
Standard curve: