Cell Cytotoxicity is a critical quality attribute (CQA) for the biological activity of immune cell therapy products (e.g., NK cells, CAR-NK cells, Tm cells, CAR-T or TCR-T cells, etc.), and it is an important element of product quality research and quality control. Compared with cell cytotoxicity methods such as lactate dehydrogenase (LDH) assay, 51Cr release assay, luciferase reporter gene assay, RTCA assay, the detection of CFSE- and 7-AAD-labelled cell killing by flow cytometry is safer, more convenient, faster and more stable. To streamline your quality control workflow, we offer a specialized cell cytotoxicity assay kit optimized for this flow cytometry-based approach, ensuring precise and reproducible results.
Dual-Label Precision: CFSE labels target cells; 7-AAD identifies lysed cells.
High Throughput: Compatible with 96-well plates.
Safety: Eliminates radioactive isotopes (vs. 51Cr).
Specification | 1 kit |
Detection Method | Flow cytometry |
Sample Type | Suspended cells |
Kit Components | CFSE, 7-AAD, lysis buffer, calibration beads |
Sensitivity | 1% cytotoxicity detectable |
| Validity period | 6 months |
| Storage conditions | 4℃/-20℃ |
| LOQ | NA |
| LOD | NA |
| IND Filing | NA |
| FDA Filing | NA |
| DMF Filing | NA |
It is suitable for the evaluation of the killing function of immune cell products such as NK cells, CAR-NK cells, CAR-T cells, TCR-T cells, Tm cells, etc. on different tumour cell lines (suspended target cells).
CMC and quality research phase: In the early development phase of cell therapy products, the cytotoxic effect is evaluated and the process is optimised.
Release testing, stability and comparability studies phase: During the mid-term process lock phase of cell therapy products, a cell killing method for the product is established, and release testing and stability testing of the cell product are performed.
| Performance Parameter | Acceptance Criteria |
| Accuracy | Recovery 70–130% |
| Robustness | Bias 70–130% |
| Repeatability | CV < 10% |
| Intermediate Precision | CV ≤ 10% |
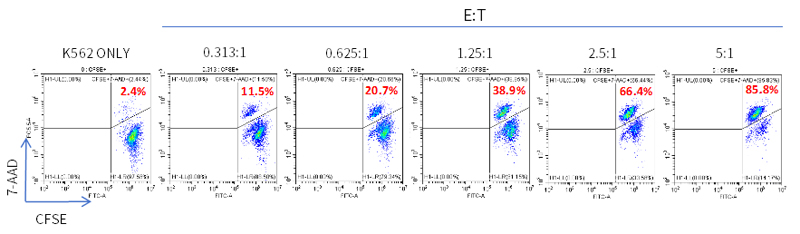
FACS analysis of the killing rate of NK cells against CFSE-labeled K562 target cells:
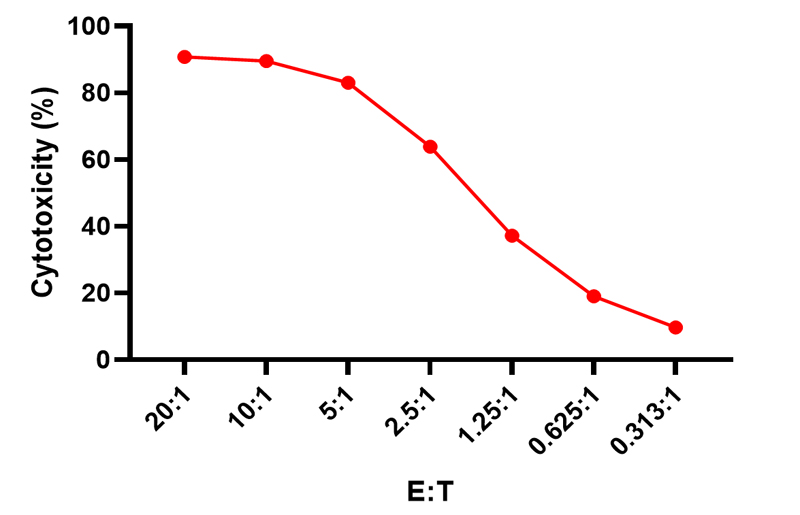
Cell Cytotoxicity curves of NK cells against CFSE-labelled K562 target cells: