BlueKit® series Kanamycin ELISA Detection Kit is a specialized kit for quantitative detection of residual kanamycin content in drug substance, intermediates, and drug products of cell and gene therapy drugs.
This kit determines the trace residue of kanamycin in samples by indirect competitive ELISA. The plate is pre-coated with conjugated antigen. Kanamycin remaining in the sample and conjugated antigen pre-coated on the plate strips compete for anti-kanamycin antibody. Add enzyme-labeled secondary antibody, and then add TMB substrate for color development. Measure the absorbance (OD value) at 450 nm/630 nm using a plate reader, and calculate the percent absorbance. The concentration of kanamycin in the sample is negatively correlated with the percent absorbance.
5X more sensitive than Thermo Fisher's kanamycin kit (0.25 ng/mL LOD)
Ready-to-use pre-coated plates save 2+ hours prep time
Included FDA 21 CFR Part 11-compliant templates
| Parameter | Specification |
| Catalog Number | IHG-KAO01 |
| Assay Range | 0.05 - 5 ng/mL |
| Limit of Detection | 0.05 ng/mL |
| Limit of Quantitation | 0.05 ng/mL |
| Format | 96-well plate |
| Storage Temperature | -14°C |
| Precision | CV% ≤15% |
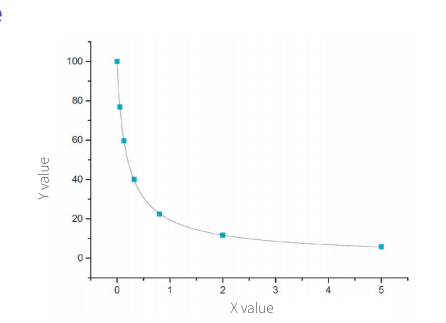
Standard Curve
Datasheet