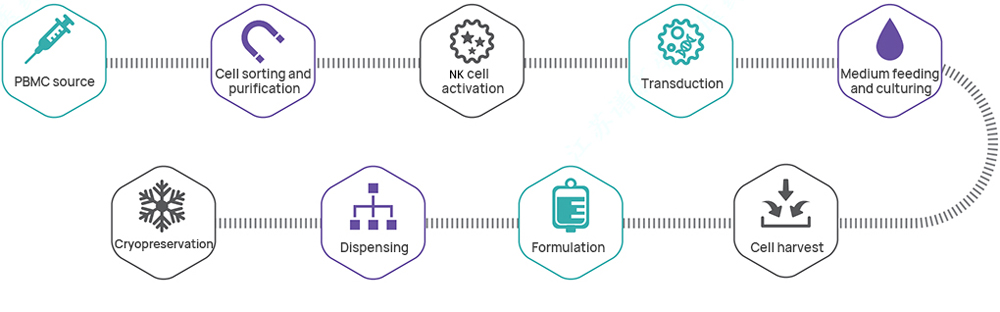
Hillgene employs a closed automated platform to provide process transfer, optimization, and IND-level production services for products such as CAR-T and TCR-T. From cell activation, transduction, and expansion to cryopreservation, every step is tightly controlled to ensure cell products exhibit high viability, high transduction efficiency, and well-defined efficacy. Our quality systems and data integrity fully meet IND application and clinical trial requirements.
| CDMO Services for CAR-NK Cells (HiCellx® Platform) | ||||
| Types | Services | |||
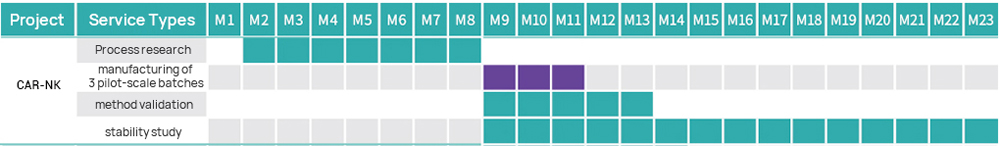
| IND grade | 1 | Process and Test Method Development | ● Following project requirements (subject to customized changes) | ● Full-GMP compliant Workshop of B+A grade with unidirectional air flow ● GMP quality management system ● Several successful submissions in China |
| 2 | GMP Manufacturing of CAR-NK Cells | ● Connecting shipment ● Production scale: 200 mL~20 L (subject to customized changes) ● Process route: flexible process design and subject to customized changes | ||
| 3 | Testing of CAR-NK Cells | ● Purity (CD3-CD56+) ● CAR positive rate ● RCL (Rapid Test) ● Number of copies ● Sterility (Compendial Method) ● Sterility (Rapid Test) ● Mycoplasma (Compendial Method) ● Mycoplasma (Rapid Test) ● Endotoxin | ||
| 4 | Method Validation | ● Specificity ● Accuracy ● Precision ● Linearity and Range ● LOD | ||
| 5 | Stability Study | ● Long-term stability ● Accelerated stability ● Stress testing ● Shipping stability | ||
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our HiCellx® technology platform:
• Using independently developed cryopreserved cell preparation
• Using closed and automated cell culturing equipment, the same as the global mainstream companies
• Cell workshop compliant with clinical and commercial requirements: grades B+A, unidirectional air flow, Full-GMP
• Cell proliferation with higher rate, solved the issues of low positive rate and proliferation rate
• Flexibly suitable for manufacturing and testing of various cellular therapy products
• Extensive experience in using the closed and automated cell culturing equipment
• Experience in manufacturing of 200+ IIT clinical samples
• Experience in IND submission of a CAR-NK cell product, which was successfully approved by NMPA
• Experience in supporting the technology transfer of clinical batch of CAR-NK cell products and in manufacturing of cell samples for clinical use
| Types | Test Item | Test Method |
| Routine tests | Appearance | Visual inspection |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Cellular characteristics/functions | Cell counts | Fluorescence staining |
| Cell viability | Fluorescence staining | |
| NK cell purity | Flow cytometry | |
| CAR positive rate | Flow cytometry | |
| Immune cell composition | Flow cytometry | |
| Cytokine secretion | ELISA | |
| Cytotoxicity | As per Protocol | |
| Impurity | Residual culture supplement | Depending on supplement type |
| Residual magnetic bead count | Microscopy | |
| Safety | Number of CAR gene copies | q-PCR |
| Endotoxin testing | Method 1143 of ChP 2020 | |
| Sterility testing | Rapid testing | |
| Method 1101 of ChP 2020 | ||
| Mycoplasma testing | q-PCR | |
| Method 3301 of ChP 2020 | ||
| RCL | q-PCR |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

As your cell therapy products (e.g., CAR-T, TCR-T, CAR-NK) progress into clinical stages, higher demands are placed on process stability, product consistency, and production scale. By optimizing process parameters and strengthening quality systems, we ensure the provision of high-quality, reproducible cell products for pivotal clinical studies (Phase II/III). We effectively manage the complexity of personalized medicines and meet the stringent consistency requirements of multi-center clinical trials.
| CDMO Services for CAR-NK Cells (HiCellx® Platform) | ||||
| Types | Services | |||
| Clinical grade | 1 | GMP Manufacturing of CAR-NK Cells | ● Production scale: 200 mL~20 L (subject to customized changes) ● Process route: flexible process design and subject to customized changes | ● Full-GMP compliant Workshop of B+A grade with unidirectional air flow ● GMP quality management system ● Involving in ongoing clinical studies |
| 2 | Technology Transfer | ● Technology transfer ● Receiving technology transfer | ● Well-established plan for technology transfer ● Well-established plan for receiving technology transfer ● Plan for transferring of different technologies across different phases | |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our HiCellx® technology platform:
• Using independently developed cryopreserved cell preparation
• Using closed and automated cell culturing equipment, the same as the global mainstream companies
• Cell workshop compliant with clinical and commercial requirements: grades B+A, unidirectional air flow, Full-GMP
• Cell proliferation with higher rate, solved the issues of low positive rate and proliferation rate
• Flexibly suitable for manufacturing and testing of various cellular therapy products
• Extensive experience in using the closed and automated cell culturing equipment
• Experience in manufacturing of 200+ IIT clinical samples
• Experience in IND submission of a CAR-NK cell product, which was successfully approved by NMPA
• Experience in supporting the technology transfer of clinical batch of CAR-NK cell products and in manufacturing of cell samples for clinical use

| Types | Test Item | Test Method |
| Routine tests | Appearance | Visual inspection |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Cellular characteristics/functions | Cell counts | Fluorescence staining |
| Cell viability | Fluorescence staining | |
| NK cell purity | Flow cytometry | |
| CAR positive rate | Flow cytometry | |
| Immune cell composition | Flow cytometry | |
| Cytokine secretion | ELISA | |
| Cytotoxicity | As per Protocol | |
| Impurity | Residual culture supplement | Depending on supplement type |
| Residual magnetic bead count | Microscopy | |
| Safety | Number of CAR gene copies | q-PCR |
| Endotoxin testing | Method 1143 of ChP 2020 | |
| Sterility testing | Rapid testing | |
| Method 1101 of ChP 2020 | ||
| Mycoplasma testing | q-PCR | |
| Method 3301 of ChP 2020 | ||
| RCL | q-PCR |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.

Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

In anticipation of diversified cell therapy commercialization, Hillgene has built a Commercial-level cell production platform renowned for its scalability, cost-efficiency, and reliability. We tackle core challenges in commercialization such as scalable production, cost control, and global supply chain stability for therapies like CAR-T and TCR-T. Through highly automated closed production systems and lean operations management, we achieve end-to-end scalable, low-cost commercial production—from patient cell collection to final product filling—ensuring every batch meets global regulatory standards.
| CDMO Services for Cell (HiCellx® Platform) | ||||
| Types | Services | |||
| Commercial grade | 1 | GMP Manufacturing of Cell Therapies | ● Production scale: 200 mL~20 L (subject to customized changes) ● Process route: flexible process design and subject to customized changes | / |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our HiCellx® technology platform:
• Using independently developed cryopreserved cell preparation
• Using closed and automated cell culturing equipment, the same as the global mainstream companies
• Cell workshop compliant with clinical and commercial requirements: grades B+A, unidirectional air flow, Full-GMP
• Cell proliferation with higher rate, solved the issues of low positive rate and proliferation rate
• Flexibly suitable for manufacturing and testing of various cellular therapy products
• Extensive experience in using the closed and automated cell culturing equipment
• Experience in manufacturing of 200+ IIT clinical samples
• Experience in IND submission of a cell therapy product, which was successfully approved by NMPA
• Experience in supporting the technology transfer of commercial batch production of a cell therapy product and in manufacturing of cell samples for clinical use

| Types | Test Item | Test Method |
| Routine tests | Appearance | Visual inspection |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Cellular characteristics/functions | Cell counts | Fluorescence staining |
| Cell viability | Fluorescence staining | |
| NK cell purity | Flow cytometry | |
| CAR positive rate | Flow cytometry | |
| Immune cell composition | Flow cytometry | |
| Cytokine secretion | ELISA | |
| Cytotoxicity | As per Protocol | |
| Impurity | Residual culture supplement | Depending on supplement type |
| Residual magnetic bead count | Microscopy | |
| Safety | Number of CAR gene copies | q-PCR |
| Endotoxin testing | Method 1143 of ChP 2020 | |
| Sterility testing | Rapid testing | |
| Method 1101 of ChP 2020 | ||
| Mycoplasma testing | q-PCR | |
| Method 3301 of ChP 2020 | ||
| RCL | q-PCR |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.

Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.
