TCR-T cells, i.e. T-cell receptor-engineered T cells, are constructed to enable T cells to specifically kill the tumor cells by inserting the TCR chains that can specifically recognize the tumor antigen into T cells through gene engineering. Hillgene is specialized in provision of integrated CDMO solutions for cellular therapy products, has established a completely closed process development platform for cellular therapy products, and therefore, can provide high-quality CDMO services for cells to clients with various demands.
Services
| CDMO Services for TCR-T Cells (HiCellx® Platform) | ||||
| Types | Services | |||
| Commercial grade | 1 | GMP Manufacturing of TCR-T Cells | ● Production scale: 200 mL~20 L (subject to customized changes) ● Process route: flexible process design and subject to customized changes | / |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages
Advantages of using our HiCellx® technology platform: • Using independently developed cryopreserved cell preparation • Using closed and automated cell culturing equipment, the same as the global mainstream companies • Cell workshop compliant with clinical and commercial requirements: grades B+A, unidirectional air flow, Full-GMP • Cell proliferation with higher rate, solved the issues of low positive rate and proliferation rate • Flexibly suitable for manufacturing and testing of various cellular therapy products • Extensive experience in using the closed and automated cell culturing equipment • Experience in manufacturing of 200+ IIT clinical samples • Experience in IND submission of a TCR-T cell product, which was successfully approved by NMPA • Experience in supporting the technology transfer of clinical batch of TCR-T cell products and in manufacturing of cell samples for clinical use |
Manufacturing process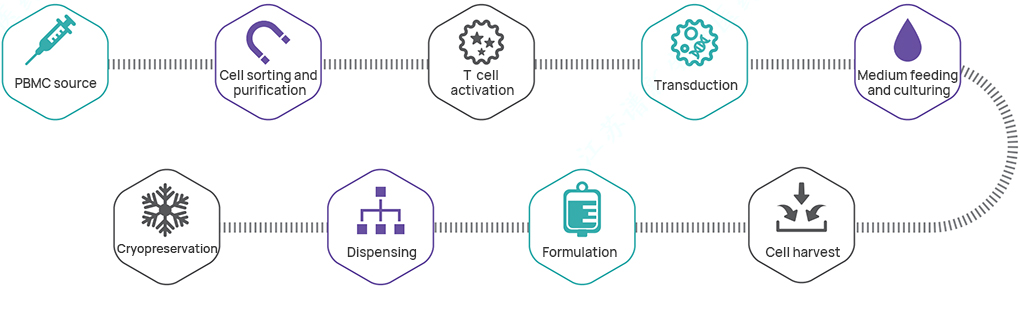
Quality control
| Types | Test Item | Test Method |
| Physicochemical Properties | Appearance | Visual inspection |
| pH | Method 0631 of ChP 2020 | |
| Purity | CD4/CD8 | Flow cytometry |
| Biological functions | Cell counts | Fluorescence staining |
| Cell viability | Fluorescence staining | |
| TCR positive rate | Flow cytometry | |
| Cytotoxicity | As per Protocol | |
| Safety | Number of TCR gene copies | q-PCR |
| Mycoplasma | q-PCR | |
| Endotoxin | Method 1143 of ChP 2020 | |
| RCL | RCL (No positive control group) | |
| Sterility | Method 1101 of ChP 2020 | |
| Sterility (Rapid Test) | Rapid testing |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Project Timeline

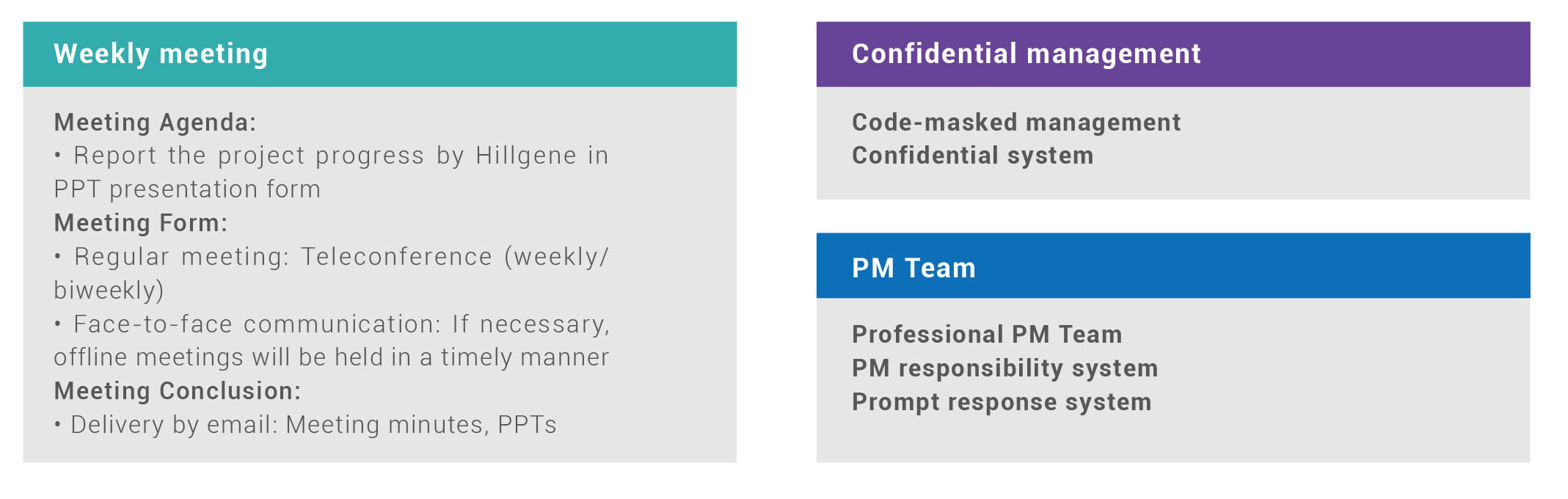
Project Management Plan
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

Building 4, Yuewang Wisdom Valley, 1463 Wuzhong Avenue, Wuzhong District, Suzhou, China