CQDMO Services (contract quality control, development, and manufacturing organization) are unique in the industry and creative service mode established by Hillgene for cellular therapy products. Given the features of the development process and our in-depth understanding of cellular therapy products, we have developed a mature QC testing protocol for cellular therapy products, thereby providing whole-process CQDMO services with excellent, multi-dimensional, and strict quality assurance.

To resolve the core technical issues of cellular therapy and accelerate the industrialization efficiency of medicine from the source, Hillgene independently researched and developed a serial of technical platforms for cellular therapy, including industrialization of nucleic acid (plasmid/mRNA), HiLenti®-T lentiviral vector system, HiLenti®-NK lentiviral vector system, HiLenti®-serum-free suspension culturing of lentiviral vectors, HiCellx®-completely closed process development for cellular products, and HiCellx® Stem-large-scale production of stem cells. With these independently developed technical platforms, we can offer our clients one-stop CDMO services for cellular therapy, thereby contributing to making more products to the next milestone sooner and faster.

Nucleic acid (plasmid/mRNA) industrialization platform, a flexible and efficient production platform for nucleic acid products, offering design plans for tailored optimization of cellular therapy products.
PLASMID INDUSTRIALIZATION PLATFORM
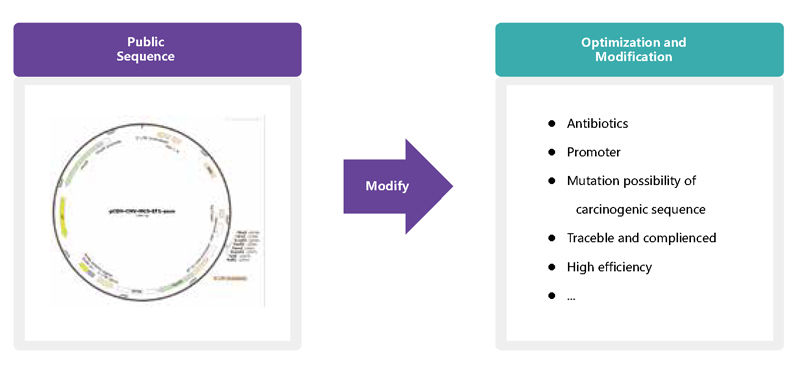
mRNA INDUSTRIALIZATION PLATFORM

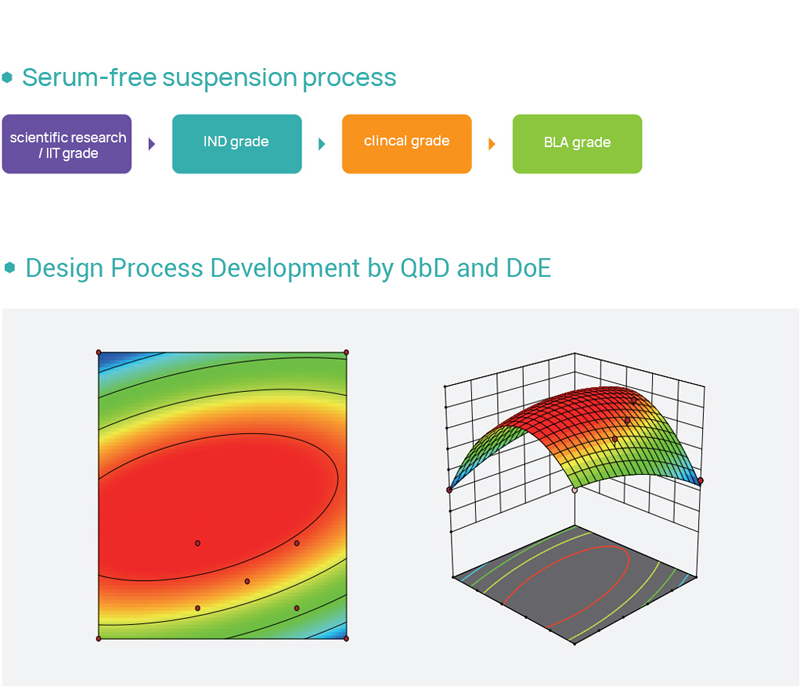
HiLenti®-T/NK lentiviral vector system, independent domestication of serum-free suspension culturing of 293 cells, using the manufacturing process of lentiviral vector in a disposable bioreactor.
HiLenti®-T/NK lentiviral vector system
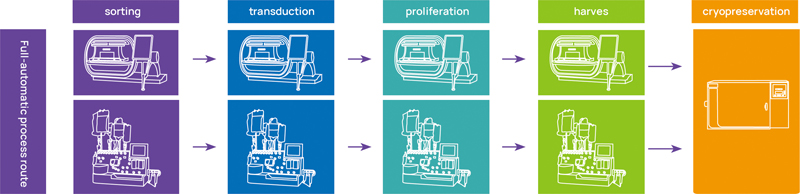
HiCellx® - completely closed process development for cellular products, equipped with closed cellular manufacturing equipment internationally accepted by all regulatory authorities, as well as the well-established closed cellular manufacturing process.
HiCellx® - completely closed process development for cellular products

Full-automatic process route
Closed or semi-closed process route
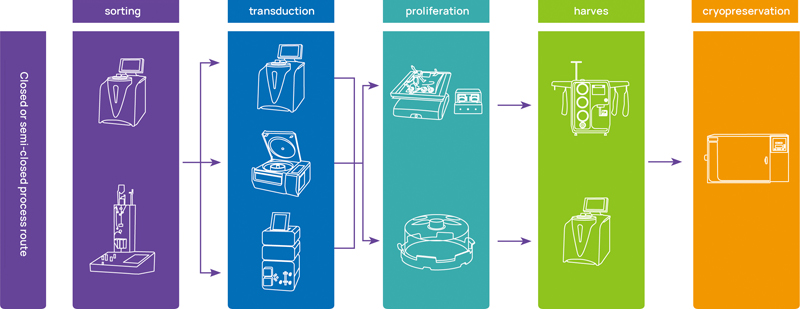
HiCellx® Stem - large-scale production of stem cells, specialized large-scale production platform for stem cells, with extensive CMC experience.
HiCellx® Stem - large-scale production of stem cells
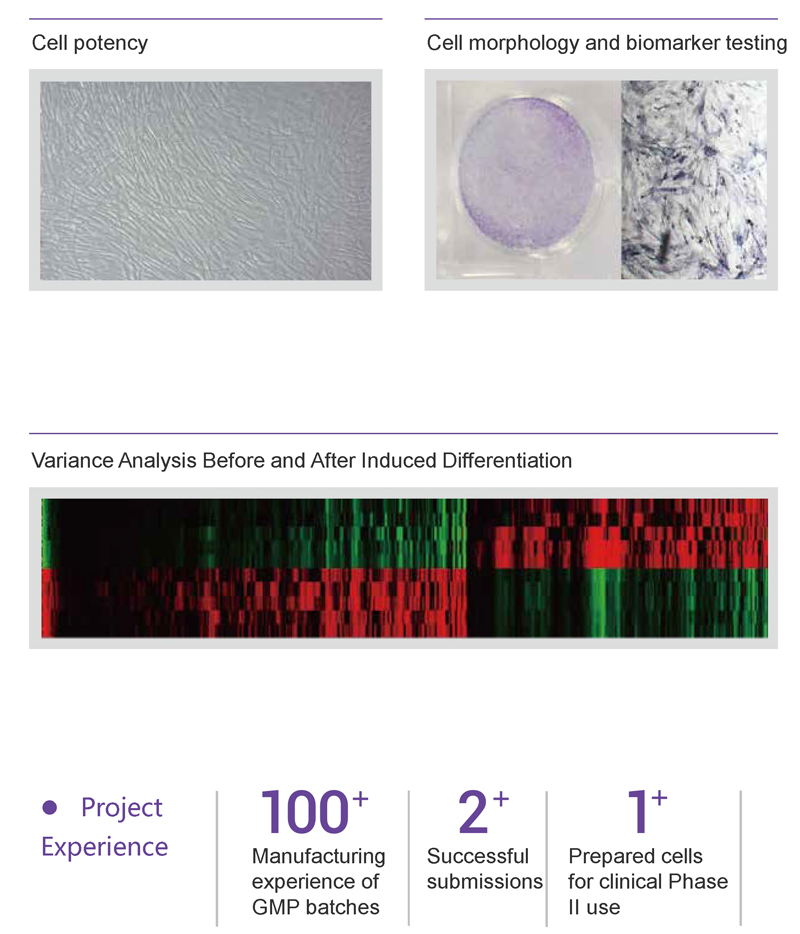
Science and regulatory consultation system, equipped with extensive experience in successful IND submissions to provide one-stop submission services for cellular therapy products.
Quality control testing platform, tailored testing methods especially for cellular therapy products, offering whole-process quality and risk control services.
Quality Control Solutions for Cellular Therapy Products
Hillgene provides the clients with testing services concerning the CDMO activities of cellular therapy products, as well as necessary supports from Discovery→IIT→IND→Clinical→BLA throughout the development period of products. Hillgene is committed to satisfaction of testing demands across different phases for the clients and provision of professional testing and quality control services, particularly for cellular and gene therapies, as well as companies involving the development and manufacturing of biological products.
Hillgene Testing Center
