This kit is designed for the detection of mycoplasma contamination in master cell bank, working cell bank, cells for clinical use and biological products. This kit conforms to relevant regulations about mycoplasma testing in EP2.6.7 and JP XVI.
This kit adopts the qPCR-fluorescent probe method. The kit is a rapid, specific and reliable device and can finish the detection within 2 hours.

100 Reactions.
| FAM(CT Value) | HEX(CT Value) | ||||||||
| Negative | Positive | Negative Positive | |||||||
| 1 | NA | 31.72 | 34.45 | 32.62 | |||||
| 2 | NA | 33.05 | 31.08 | 33.12 | |||||
| 3 | NA | 36.32 | 33.43 | 33.45 | |||||
| 4 | NA | 30.01 | 34.56 | 30.02 | |||||
| 5 | NA | 30.37 | 35.05 | 33.55 | |||||
| 6 | NA | 34.68 | 34.22 | 34.19 | |||||
| 7 | NA | 34.83 | 34.35 | 32.63 | |||||
| 8 | NA | 39.23 | 34.12 | 32.58 | |||||
| 9 | NA | 32.95 | 33.39 | 34.07 | |||||
| 10 | NA | 37.44 | 33.41 | 32.24 | |||||
| Results of 10 mycoplasma standards | Results of 3 relevant bacteria | ||||||||
| Strain | Positive/Total | Strain | Positive/Total | L.acidophilus | S.pneumoniae | Clos. acetobutyleum | |||
| M.orale | 24/24 | M.synoviae | 24/24 | Negative | Negative | Negative | |||
| M.galliscepticum | 23/24 | M.arginini | 23/24 | ||||||
| A.laidlawii | 24/24 | M.hyorhinis | 24/24 | ||||||
| M.fermentans | 23/24 | Spiroplasma citri | 24/24 | ||||||
| M.pneumonia | 24/24 | M.saliuarium | 24/24 | ||||||
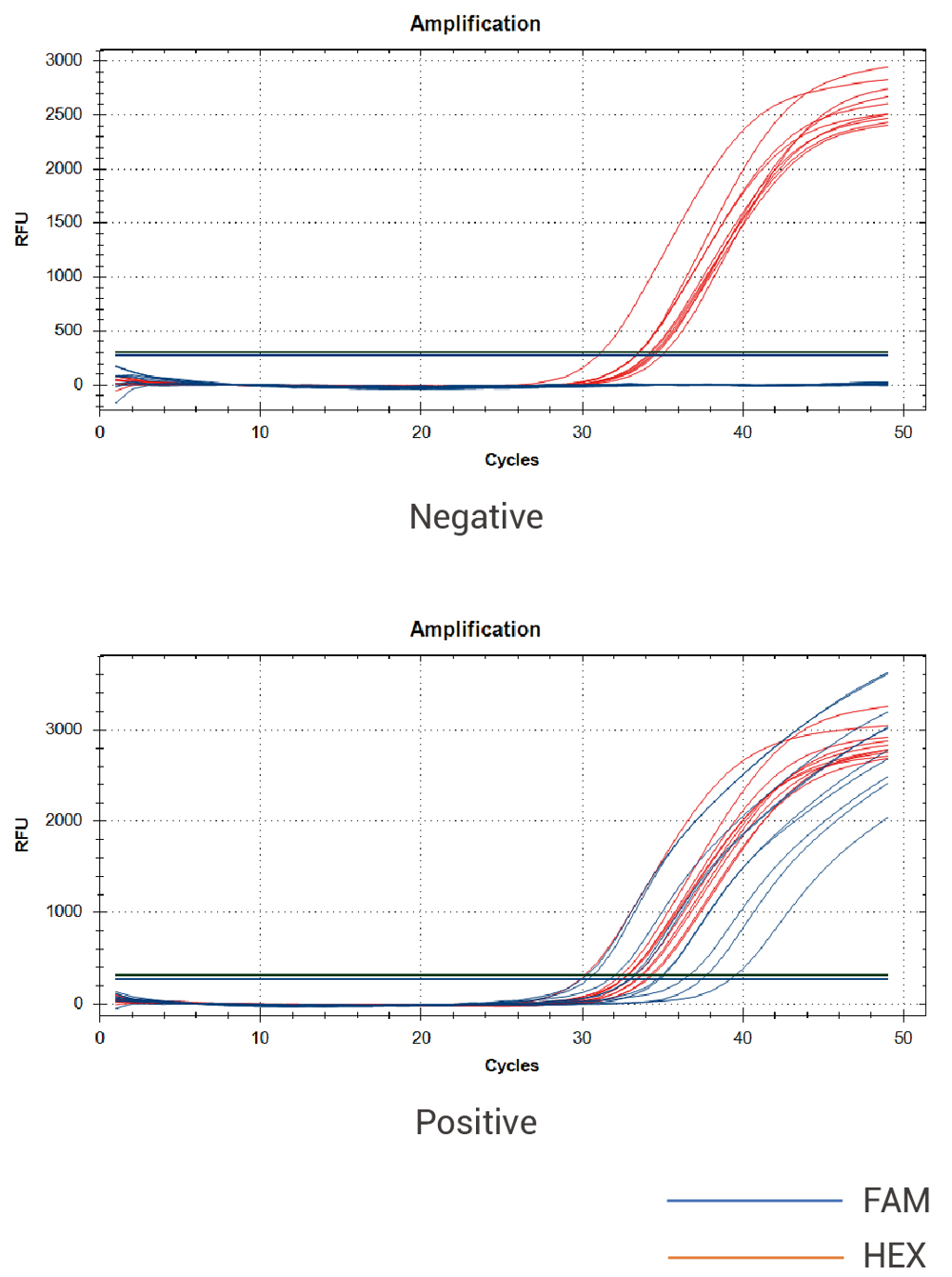
Test curve: