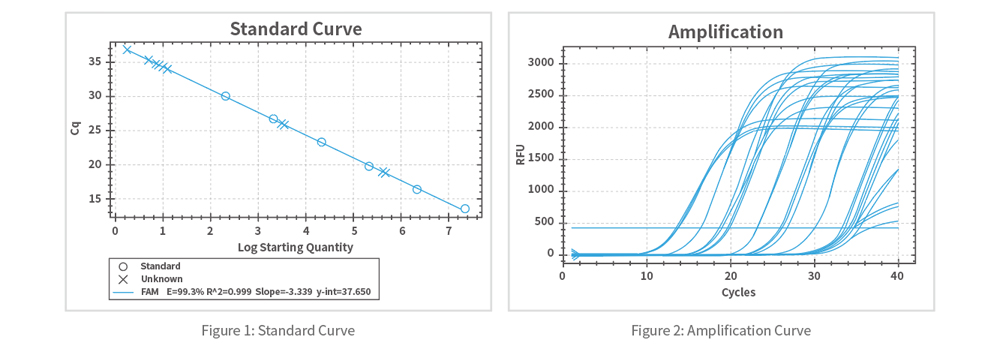
This kit utilizes fluorescently labeled specific probes or dyes that bind to specific sequences in lentiviral nucleic acids. During PCR amplification, real-time monitoring of DNA amplification is achieved by detecting changes in fluorescence signals, enabling accurate determination of lentiviral nucleic acid copy numbers and thereby quantifying lentiviral titers.
Detection Method: This kit employs the TaqMan fluorescent probe-based qPCR method to detect vector RNA copy numbers for lentiviral titer determination.
Application Scenarios: Suitable for lentiviral testing, process development, process validation, production release, and stability studies.

Target design located in the conserved LTR region, compatible with all current lentiviral vector backbones.
Calibrated using NIBSC/WHO (18/132Q) standards for reliable results.
Dual DNase digestion effectively eliminates interference from residual plasmid DNA.
Color-coded components to prevent usage errors.
The detection kit has been validated according to pharmacopeial standards for specificity, accuracy, precision, linear range, limit of quantification (LOQ), limit of detection (LOD), robustness, and stability. Production release is traceable and quality-controlled.
Dedeicated product specialists for streamlined communication.
24-hour response mechanism for immediate issue resolution.
| Specification | 100 Reactions |
| Validity period | 18 months |
| Storage conditions | -20℃ |
| LOQ | 200 copies/μL |
| LOD | 5copies/μL |
| Batch | p24 particles(PP/mL) | Titer (TU/mL) | Titer (TU/mL) | Ratio (vg/TU) | Ratio (PP/TU) |
| LV-A | 1.90E+10 | 2.00E+07 | 2.00E+07 | 345 | 950 |
| LV-B | 2.70E+10 | 2.90E+07 | 2.90E+07 | 347 | 931 |
| LV-C | 3.40E+10 | 3.70E+07 | 3.70E+07 | 324 | 919 |
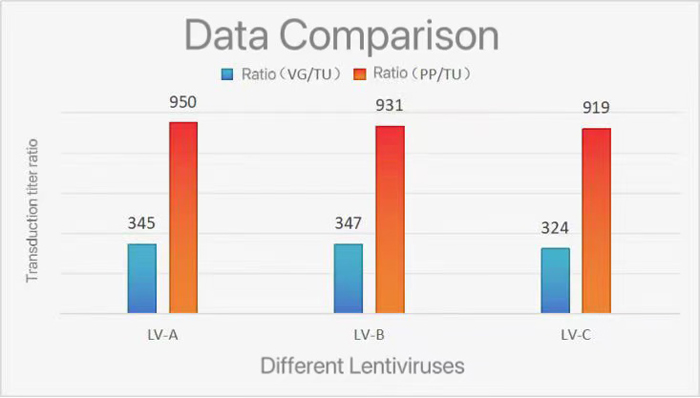
Data from Figure above indicate that viral particle counts detected using Vector RNA Copy Number are significantly closer to transduction titer results compared to p24-based detection. Lentivirus Vector RNA Copy Number Detection Kit provides more accurate measurement of lentiviral physical titers.