Manufacturing of plasmids, a critical step of manufacturing CAR-NK cellular therapy products, involves a series of complicated processes of manufacturing, purification, and analysis. As essential tools for genetic engineering, bacterial plasmids can not only be used as final products for gene and cellular therapy, but also as intermediate vectors for the manufacturing of gene and cell therapy products, and are inevitably used in manufacturing steps for most gene and cellular therapy products. With the emergence of the cellular therapy industry, the market demands for plasmids are also increasing with each passing year. Hillgene is specialized in the provision of integrated CDMO solutions for cellular therapy products, has established a GMP manufacturing platform for nucleic acid products, and therefore, can provide high-quality CDMO services for plasmids to clients with various demands.
Services
| CDMO Services for Plasmids | ||||
| Types | Services | |||
| Commercial grade | 1 | GMP Manufacturing of Plasmids | ● Production output: 10 mg~1 g (subject to customized changes) ● Fermentation volume: 3~30 L (subject to customized changes) ● Purification method: three-step approach/two-step approach | / |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages
| Advantages of our plasmid system: • An independently developed four-plasmid system with kanamycin-resistance gene • A system with the capability of sustained optimization • Plasmid sequences are traceable, compliant with requirements, and efficient • Extensive experience in successful IND submissions • CAR-T cell samples for clinical use are currently manufacturing and in use • 2-5 folds higher titers after using our plasmid system from the comparison in several projects | Advantages of our plasmid manufacturing: • Free of antibiotics throughout the manufacturing process • Plasmid production and bank creation in separate workshops • Complete isolation between non-sterile and sterile areas • Dispensing final products using an isolator • Completed CTD dossiers for packaging plasmid (for lentiviral vector), reducing the submission preparation time by 3-4 months, with INDs of a few products granted preliminary approval and currently in phase I of clinical study |
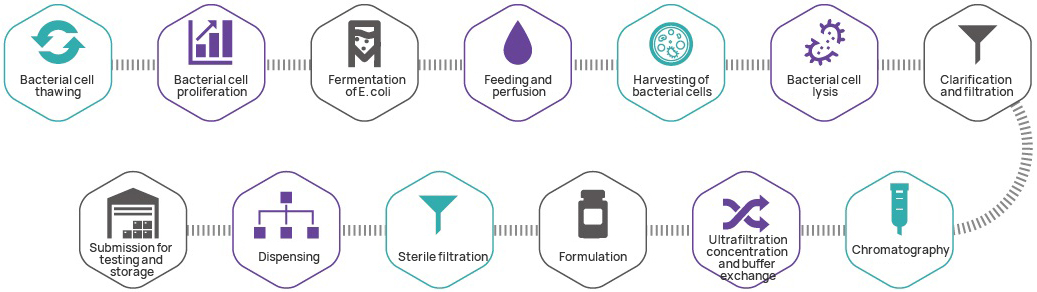
Manufacturing process
Quality control
| Test Item | Test Method | |
| Appearance | Visual inspection | |
| Identification | Identification 1 | Restriction mapping |
| Identification 2 | Sanger sequencing | |
| Test | pH | Method 0631 of ChP 2020 |
| Purity | High performance liquid chromatography (HPLC) | |
| Residual E.coli host cell protein | ELISA | |
| Residual E.coli DNA | q-PCR | |
| Residual E.coli RNA | q-PCR | |
Residual antibiotics | ELISA | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Sterility | Method 1101 of ChP 2020 | |
| Concentration determination | DNA concentration | Method 0401 of ChP 2020 |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Project Timeline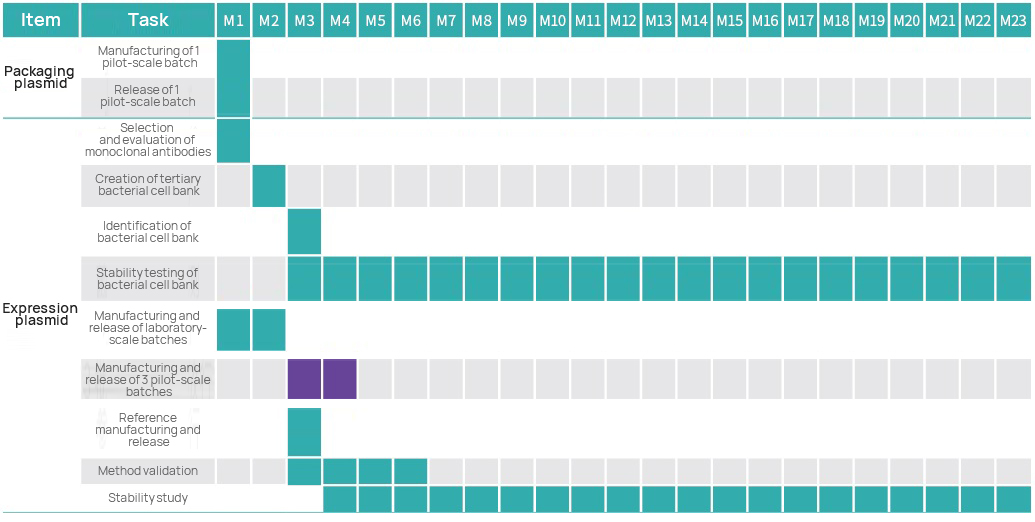
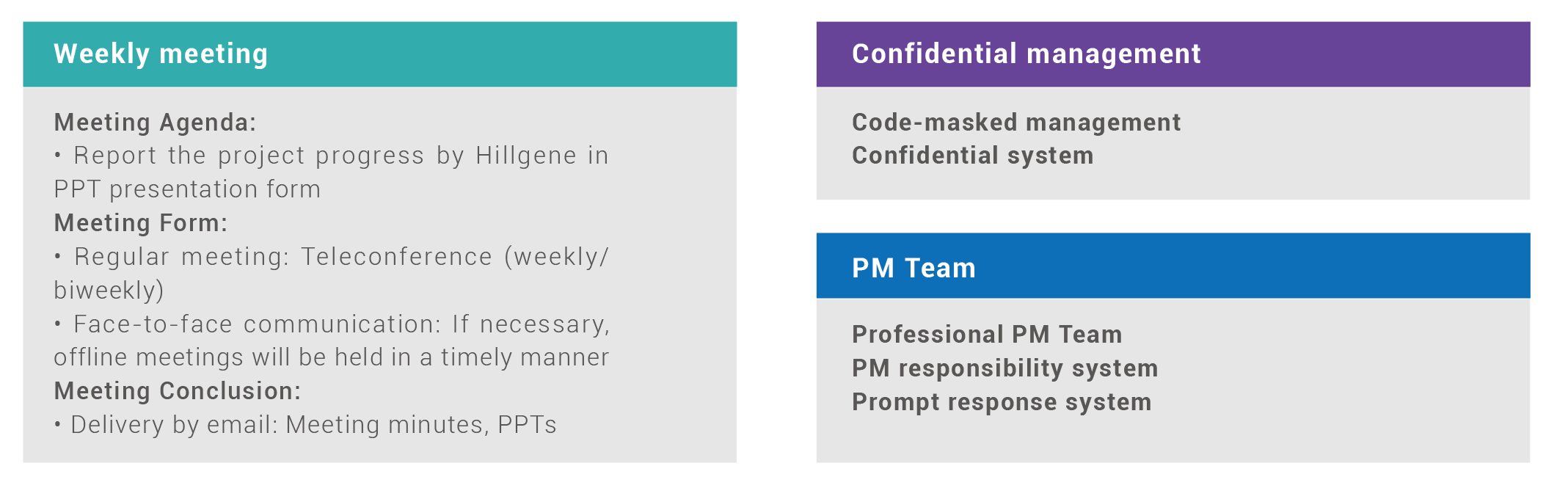
Project Management Plan
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

Building 4, Yuewang Wisdom Valley, 1463 Wuzhong Avenue, Wuzhong District, Suzhou, China