Hillgene offers end-to-end lentiviral vector CDMO services, delivering high-quality, scalable, and regulatory-compliant solutions for cell and gene therapy developers. From initial plasmid design and vector construction to GMP-grade manufacturing and rigorous quality control testing, our services support your program across all stages. With extensive experience in lentivirus production and a strong focus on viral safety and process consistency, Hillgene ensures:
Accelerated timelines
Flexible batch sizes
Cost-effective production options

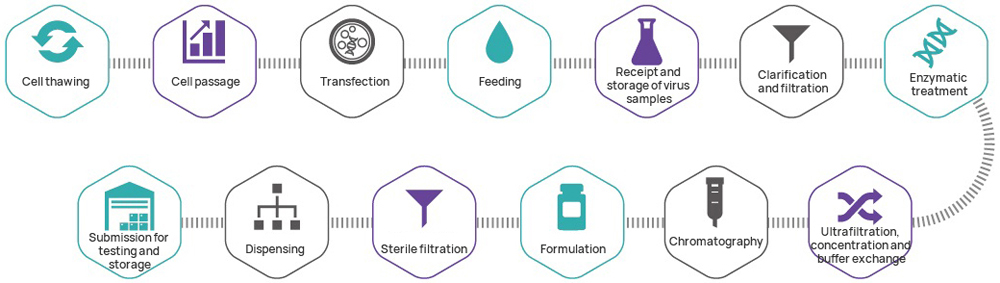
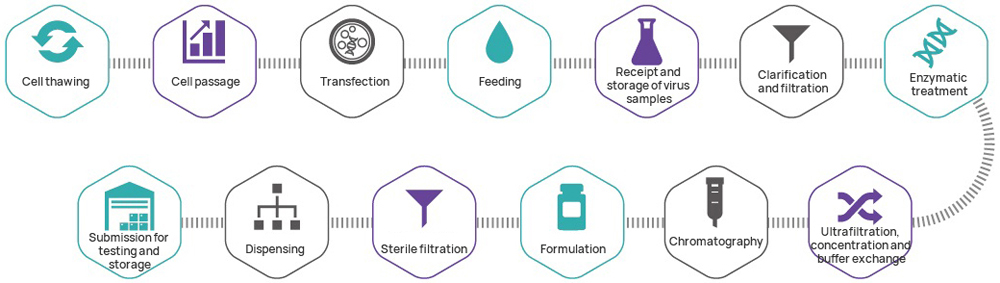
Hillgene can provide the following lentiviral vector CDMO services at different stages

We specialize in both research-grade and clinical-grade lentiviral vectors tailored to each client’s needs. Our state-of-the-art facilities support CAR-T, CAR-NK, and other advanced cell therapy applications. As a trusted CDMO partner, Hillgene operates under strict GMP guidelines and aligns with global regulatory standards. Partner with Hillgene to accelerate your gene therapy pipeline with reliable, scalable, and cost-efficient lentiviral vector manufacturing.
Self-adapted third-generation quad plasmid vector system
With traditional VSVG and novel envelope
Completed DMF filing
Virus Expressed 293T serum-free culture technology
Disposable bioreactors
Yield: Up to 2E11TU/50L
Scale: From 10L up to 100L
Different GOI lengths, at a lower MOI, can obtain a high positive rate, low copy number of the cell products

Can be applied to different cells
CAR-T therapy for Solid tumors
TCR-T cell therapeutics
Gene-edited HSCs
UCAR-T cell therapeutics
CAR-NK cell therapeutics
iPS-CAR -NK cell therapeutics
At Hillgene, we leverage our advanced serum-free suspension culture GMP platform to provide lentiviral vector CDMO services that meet IND application requirements, with products directly suitable for cell transduction in clinical trials. Our end-to-end process—from vector construction and virus packaging to purification and testing—complies with pharmaceutical regulatory standards. The delivered viral solutions exhibit high titer, high infectivity, and an excellent safety profile (including rigorous RCL testing), ensuring the safe and efficient production of cell-based products such as CAR-T and CAR-NK for clinical trials.
| CDMO Services for Lentiviral Vectors (HiLenti® Platform) | ||||
| Types | Services | |||
| IND grade | 1 | Independently developed four-plasmid system | ● Third generation four-plasmid system ● Granting the license, if required | ● Following standards for submission in both China and US ● Full-GMP workshop ● Separate area for creating cell banks ● Separate workshops within non-sterile and sterile areas ● GMP quality management system |
| 2 | Creation of GMP Cell Bank | ● Tailorable number of cell banks to be created ● Cell bank stability study | ||
| 3 | Process and Test Method Development | ● Following project requirements (subject to customized changes) | ||
| 4 | GMP Manufacturing of Lentiviral Vectors | ● Bioreactor process: 5~50 L disposable bioreactor process (subject to customized changes) ● Production scale: 2~30 L (subject to customized changes) | ||
| 5 | Testing of Lentiviral Vectors | ● Physical titer ● Infective titer ● Functional titer ● Residual 293T host cell DNA testing ● Residual 293T host cell protein testing ● Residual exogenous DNA testing ● Residual Benzonase testing ● E1A/SV40 ● Residual plasmid testing ● DNA fragment size ● Exogenous virokines ● Sterility ● Mycoplasma ● Endotoxin | ||
| 6 | Method Validation | ● Specificity ● Accuracy ● Precision ● Linearity and Range ● LOD | ||
| 7 | Stability Study | ● Long-term stability ● Accelerated stability ● Stress testing | ||
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our platform for serum-free suspension culturing of lentiviral vectors:
• Free of animal-derived components throughout the process
• Linearly scaled up production of lentiviral vectors
• Using a single container of a 50 L disposable bioreactor
• Cell bank creation in separate workshops
• Dispensing final products using a sterile isolator
• Dedicated lentivirus system for cells, with high infection efficiency
• Low production costs and testing costs (no requirements of testing for BSA and residual pancreatic enzymes)
• Several successful IND submissions to NMPA of lentiviral vectors for cells
| Product | Test Item | Test Method |
| Harvest Fluid | Adventitious virus contamination | Method 3302 of ChP 2020 |
| Replication-competent lentiviruses | Indicator cell culture method | |
| Drug substance/finished product | Appearance | Visual inspection |
| Sterility | Method 1101 of ChP 2020 | |
| Mycoplasma | Method 3301 of ChP 2020 | |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Target gene structure identification | Sequencing | |
| Residual host cell protein | ELISA | |
| Physical titer (p24) | ELISA | |
| Functional titer | Flow cytometry | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Residual Benzonase | ELISA | |
| Residual host cell DNA | q-PCR | |
| Residual E1A gene transfer | Co-culture method | |
| Residual SV40 gene transfer | Co-culture method |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

At this stage, Hillgene offers a comprehensively upgraded Clinical-level CDMO solution. Through process scale-up and enhanced quality systems, we ensure stable supply of highly consistent clinical-grade viral materials for Phase II/III clinical trials. This fully meets the medication needs of multi-center trials and diverse patient populations, supporting smooth clinical development. Our entire production process operates in a cGMP environment, adhering to international standards with full traceability. We are committed to ensuring patient safety and trial progress, serving as your trusted partner in clinical development.
| CDMO Services for Lentiviral Vectors (HiLenti® Platform) | ||||
| Types | Services | |||
| Clinical grade | 1 | GMP Manufacturing of Lentiviral Vectors | ● Bioreactor process: 5~50 L disposable bioreactor process (subject to customized changes) ● Production scale: 2~30 L (subject to customized changes) | ● Full-GMP workshop ● Separate workshops within non-sterile and sterile areas ● GMP quality management system ● Validated plant, facility and equipment compliant with clinical requirements |
| 2 | Technology Transfer | ● Technology transfer ● Receiving technology transfer | ● Well-established plan for technology transfer ● Well-established plan for receiving technology transfer ● Plan for transferring different technologies across different phases | |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our platform for serum-free suspension culturing of lentiviral vectors:
• Free of animal-derived components throughout the process
• Linearly scaled up production of lentiviral vectors
• Using a single container of a 50 L disposable bioreactor
• Cell bank creation in separate workshops
• Dispensing final products using a sterile isolator
• Dedicated lentivirus system for cells, with high infection efficiency
• Low production costs and testing costs (no requirements of testing for BSA and residual pancreatic enzymes)
• Several successful IND submissions to NMPA of lentiviral vectors for cells
| Product | Test Item | Test Method |
| Harvest Fluid | Adventitious virus contamination | Method 3302 of ChP 2020 |
| Replication-competent lentiviruses | Indicator cell culture method | |
| Drug substance/finished product | Appearance | Visual inspection |
| Sterility | Method 1101 of ChP 2020 | |
| Mycoplasma | Method 3301 of ChP 2020 | |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Target gene structure identification | Sequencing | |
| Residual host cell protein | ELISA | |
| Physical titer (p24) | ELISA | |
| Functional titer | Flow cytometry | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Residual Benzonase | ELISA | |
| Residual host cell DNA | q-PCR | |
| Residual E1A gene transfer | Co-culture method | |
| Residual SV40 gene transfer | Co-culture method |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

Looking toward the commercialization of cell therapies, Hillgene has established a Commercial-level lentiviral production platform with high cost-effectiveness and significant production capacity. We focus on addressing two core challenges of the commercialization stage: cost reduction and stable supply. Through continuous process optimization and smart manufacturing, we achieve large-scale (thousands of liters), low-cost, high-titer lentiviral vector production. Every batch strictly complies with global regulatory requirements.
| CDMO Services for Lentiviral Vectors (HiLenti® Platform) | ||||
| Types | Services | |||
| Commercial grade | 1 | GMP Manufacturing of Lentiviral Vectors | ● Bioreactor process: 5~50 L disposable bioreactor process (subject to customized changes) ● Production scale: 2~30 L (subject to customized changes) | / |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our platform for serum-free suspension culturing of lentiviral vectors:
• Free of animal-derived components throughout the process
• Linearly scaled up production of lentiviral vectors
• Using a single container of a 50 L disposable bioreactor
• Cell bank creation in separate workshops
• Dispensing final products using a sterile isolator
• Dedicated lentivirus system for cells, with high infection efficiency
• Low production costs and testing costs (no requirements of testing for BSA and residual pancreatic enzymes)
• Several successful IND submissions to NMPA of lentiviral vectors for cells

| Product | Test Item | Test Method |
| Harvest Fluid | Adventitious virus contamination | Method 3302 of ChP 2020 |
| Replication-competent lentiviruses | Indicator cell culture method | |
| Drug substance/finished product | Appearance | Visual inspection |
| Sterility | Method 1101 of ChP 2020 | |
| Mycoplasma | Method 3301 of ChP 2020 | |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Target gene structure identification | Sequencing | |
| Residual host cell protein | ELISA | |
| Physical titer (p24) | ELISA | |
| Functional titer | Flow cytometry | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Residual Benzonase | ELISA | |
| Residual host cell DNA | q-PCR | |
| Residual E1A gene transfer | Co-culture method | |
| Residual SV40 gene transfer | Co-culture method |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.

Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

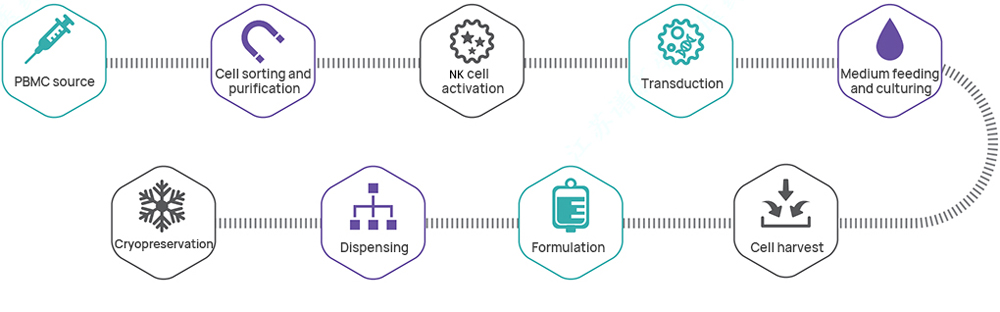
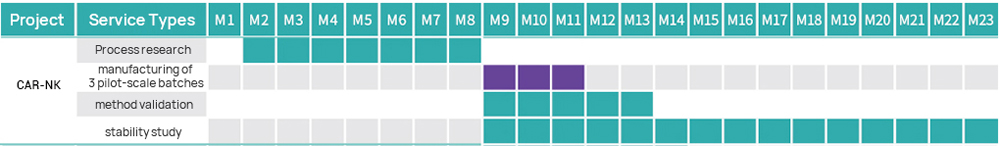
Hillgene employs a closed automated platform to provide process transfer, optimization, and IND-level production services for products such as CAR-T and TCR-T. From cell activation, transduction, and expansion to cryopreservation, every step is tightly controlled to ensure cell products exhibit high viability, high transduction efficiency, and well-defined efficacy. Our quality systems and data integrity fully meet IND application and clinical trial requirements.
| CDMO Services for CAR-NK Cells (HiCellx® Platform) | ||||
| Types | Services | |||
| IND grade | 1 | Process and Test Method Development | ● Following project requirements (subject to customized changes) | ● Full-GMP compliant Workshop of B+A grade with unidirectional air flow ● GMP quality management system ● Several successful submissions in China |
| 2 | GMP Manufacturing of CAR-NK Cells | ● Connecting shipment ● Production scale: 200 mL~20 L (subject to customized changes) ● Process route: flexible process design and subject to customized changes | ||
| 3 | Testing of CAR-NK Cells | ● Purity (CD3-CD56+) ● CAR positive rate ● RCL (Rapid Test) ● Number of copies ● Sterility (Compendial Method) ● Sterility (Rapid Test) ● Mycoplasma (Compendial Method) ● Mycoplasma (Rapid Test) ● Endotoxin | ||
| 4 | Method Validation | ● Specificity ● Accuracy ● Precision ● Linearity and Range ● LOD | ||
| 5 | Stability Study | ● Long-term stability ● Accelerated stability ● Stress testing ● Shipping stability | ||
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our HiCellx® technology platform:
• Using independently developed cryopreserved cell preparation
• Using closed and automated cell culturing equipment, the same as the global mainstream companies
• Cell workshop compliant with clinical and commercial requirements: grades B+A, unidirectional air flow, Full-GMP
• Cell proliferation with higher rate, solved the issues of low positive rate and proliferation rate
• Flexibly suitable for manufacturing and testing of various cellular therapy products
• Extensive experience in using the closed and automated cell culturing equipment
• Experience in manufacturing of 200+ IIT clinical samples
• Experience in IND submission of a CAR-NK cell product, which was successfully approved by NMPA
• Experience in supporting the technology transfer of clinical batch of CAR-NK cell products and in manufacturing of cell samples for clinical use
| Types | Test Item | Test Method |
| Routine tests | Appearance | Visual inspection |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Cellular characteristics/functions | Cell counts | Fluorescence staining |
| Cell viability | Fluorescence staining | |
| NK cell purity | Flow cytometry | |
| CAR positive rate | Flow cytometry | |
| Immune cell composition | Flow cytometry | |
| Cytokine secretion | ELISA | |
| Cytotoxicity | As per Protocol | |
| Impurity | Residual culture supplement | Depending on supplement type |
| Residual magnetic bead count | Microscopy | |
| Safety | Number of CAR gene copies | q-PCR |
| Endotoxin testing | Method 1143 of ChP 2020 | |
| Sterility testing | Rapid testing | |
| Method 1101 of ChP 2020 | ||
| Mycoplasma testing | q-PCR | |
| Method 3301 of ChP 2020 | ||
| RCL | q-PCR |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

As your cell therapy products (e.g., CAR-T, TCR-T, CAR-NK) progress into clinical stages, higher demands are placed on process stability, product consistency, and production scale. By optimizing process parameters and strengthening quality systems, we ensure the provision of high-quality, reproducible cell products for pivotal clinical studies (Phase II/III). We effectively manage the complexity of personalized medicines and meet the stringent consistency requirements of multi-center clinical trials.
| CDMO Services for CAR-NK Cells (HiCellx® Platform) | ||||
| Types | Services | |||
| Clinical grade | 1 | GMP Manufacturing of CAR-NK Cells | ● Production scale: 200 mL~20 L (subject to customized changes) ● Process route: flexible process design and subject to customized changes | ● Full-GMP compliant Workshop of B+A grade with unidirectional air flow ● GMP quality management system ● Involving in ongoing clinical studies |
| 2 | Technology Transfer | ● Technology transfer ● Receiving technology transfer | ● Well-established plan for technology transfer ● Well-established plan for receiving technology transfer ● Plan for transferring of different technologies across different phases | |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our HiCellx® technology platform:
• Using independently developed cryopreserved cell preparation
• Using closed and automated cell culturing equipment, the same as the global mainstream companies
• Cell workshop compliant with clinical and commercial requirements: grades B+A, unidirectional air flow, Full-GMP
• Cell proliferation with higher rate, solved the issues of low positive rate and proliferation rate
• Flexibly suitable for manufacturing and testing of various cellular therapy products
• Extensive experience in using the closed and automated cell culturing equipment
• Experience in manufacturing of 200+ IIT clinical samples
• Experience in IND submission of a CAR-NK cell product, which was successfully approved by NMPA
• Experience in supporting the technology transfer of clinical batch of CAR-NK cell products and in manufacturing of cell samples for clinical use

| Types | Test Item | Test Method |
| Routine tests | Appearance | Visual inspection |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Cellular characteristics/functions | Cell counts | Fluorescence staining |
| Cell viability | Fluorescence staining | |
| NK cell purity | Flow cytometry | |
| CAR positive rate | Flow cytometry | |
| Immune cell composition | Flow cytometry | |
| Cytokine secretion | ELISA | |
| Cytotoxicity | As per Protocol | |
| Impurity | Residual culture supplement | Depending on supplement type |
| Residual magnetic bead count | Microscopy | |
| Safety | Number of CAR gene copies | q-PCR |
| Endotoxin testing | Method 1143 of ChP 2020 | |
| Sterility testing | Rapid testing | |
| Method 1101 of ChP 2020 | ||
| Mycoplasma testing | q-PCR | |
| Method 3301 of ChP 2020 | ||
| RCL | q-PCR |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.

Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

In anticipation of diversified cell therapy commercialization, Hillgene has built a Commercial-level cell production platform renowned for its scalability, cost-efficiency, and reliability. We tackle core challenges in commercialization such as scalable production, cost control, and global supply chain stability for therapies like CAR-T and TCR-T. Through highly automated closed production systems and lean operations management, we achieve end-to-end scalable, low-cost commercial production—from patient cell collection to final product filling—ensuring every batch meets global regulatory standards.
| CDMO Services for Cell (HiCellx® Platform) | ||||
| Types | Services | |||
| Commercial grade | 1 | GMP Manufacturing of Cell Therapies | ● Production scale: 200 mL~20 L (subject to customized changes) ● Process route: flexible process design and subject to customized changes | / |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of using our HiCellx® technology platform:
• Using independently developed cryopreserved cell preparation
• Using closed and automated cell culturing equipment, the same as the global mainstream companies
• Cell workshop compliant with clinical and commercial requirements: grades B+A, unidirectional air flow, Full-GMP
• Cell proliferation with higher rate, solved the issues of low positive rate and proliferation rate
• Flexibly suitable for manufacturing and testing of various cellular therapy products
• Extensive experience in using the closed and automated cell culturing equipment
• Experience in manufacturing of 200+ IIT clinical samples
• Experience in IND submission of a cell therapy product, which was successfully approved by NMPA
• Experience in supporting the technology transfer of commercial batch production of a cell therapy product and in manufacturing of cell samples for clinical use

| Types | Test Item | Test Method |
| Routine tests | Appearance | Visual inspection |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Cellular characteristics/functions | Cell counts | Fluorescence staining |
| Cell viability | Fluorescence staining | |
| NK cell purity | Flow cytometry | |
| CAR positive rate | Flow cytometry | |
| Immune cell composition | Flow cytometry | |
| Cytokine secretion | ELISA | |
| Cytotoxicity | As per Protocol | |
| Impurity | Residual culture supplement | Depending on supplement type |
| Residual magnetic bead count | Microscopy | |
| Safety | Number of CAR gene copies | q-PCR |
| Endotoxin testing | Method 1143 of ChP 2020 | |
| Sterility testing | Rapid testing | |
| Method 1101 of ChP 2020 | ||
| Mycoplasma testing | q-PCR | |
| Method 3301 of ChP 2020 | ||
| RCL | q-PCR |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.

Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

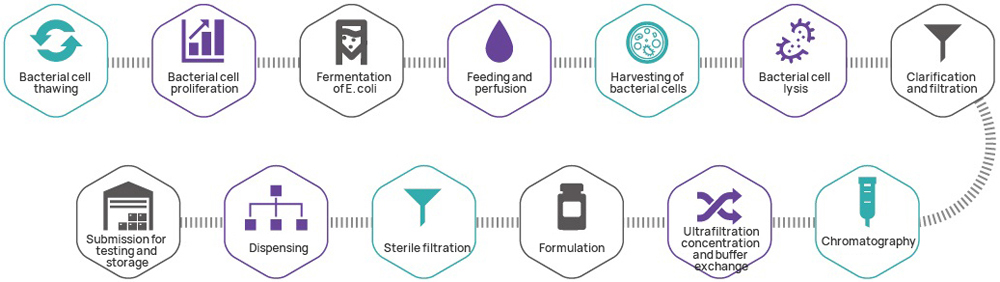
As the genetic starting point for most cell and gene therapy products, plasmids are critical. Leveraging its GMP platform for nucleic acid therapeutics, Hillgene operates as a dedicated plasmid CDMO specializing in high-copy-number seed bank construction, high-density fermentation process development, and high-resolution purification methods. Our IND-level plasmid production system strictly follows GMP standards, yielding clinical-grade plasmids directly applicable for investigator-initiated trials (IIT) and IND submissions.
| CDMO Services for Plasmids | ||||
| Types | Services | |||
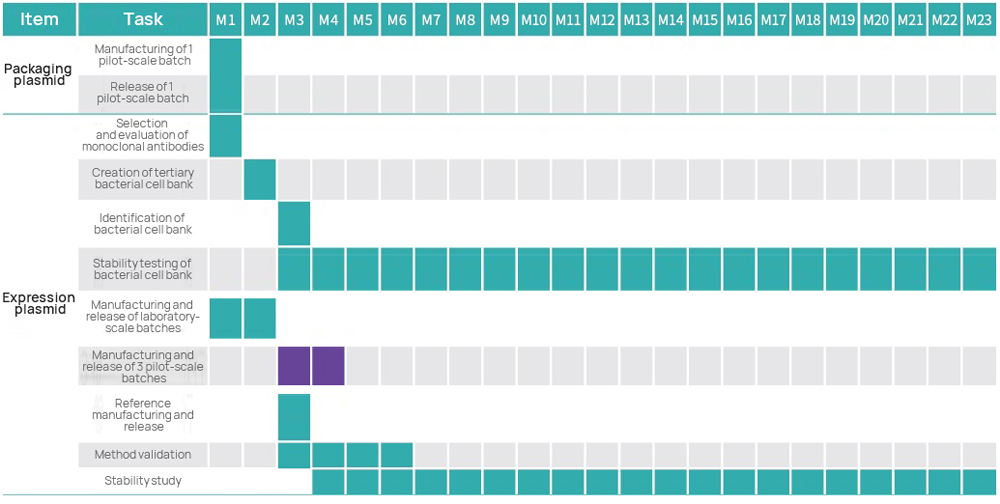
| IND grade | 1 | Independently Developed Four-Plasmid System | ● Third generation four-plasmid system ● KanR ● Granting the license, if required | ● Following standards for submission in both China and US ● Full-GMP workshop ● Separate area for creating cell banks ● Separate workshops within non-sterile and sterile areas ● GMP quality management system |
| 2 | GMP Creation of Bacterial Cell Bank | ● Selection of monoclonal antibodies ● Tailorable number of cell banks to be created ● Cell bank stability study | ||
| 3 | Process and Test Method Development | ● Following project requirements (subject to customized changes) | ||
| 4 | GMP Manufacturing of Plasmids | ● Production output: 10 mg~1 g (subject to customized changes) ● Fermentation volume: 3~30 L (subject to customized changes) ● Purification method: three-step approach/two-step approach | ||
| 5 | Plasmid Testing | ● Purity (HPLC) ● Residual E.coli DNA testing ● Residual E.coli HCP testing ● Residual E.coli RNA testing ● Residual antibiotics testing ● Sterility ● Mycoplasma ● Endotoxin | ||
| 6 | Method Validation | ● Specificity ● Accuracy ● Precision ● Linearity and Range ● LOD | ||
| 7 | Stability Study | ● Long-term stability ● Accelerated stability ● Stress testing | ||
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of our plasmid system:
• An independently developed four-plasmid system with KanR
• A system with the capability of sustained optimization
• Plasmid sequences are traceable, compliant with requirements, and efficient
• Extensive experience in successful IND submissions
• TCR-T cell samples for clinical use are currently manufacturing and in use
• 2-5 folds higher titers after using our plasmid system from the comparison in several projects
Advantages of our plasmid manufacturing:
• Free of antibiotics throughout the manufacturing process
• Plasmid production and bank creation in separate workshops
• Complete isolation between non-sterile and sterile areas
• Dispensing final products using an isolator
• Completed CTD dossiers for packaging plasmid (for lentiviral vector), reducing the submission preparation time by 3-4 months, with INDs of a few products granted preliminary approval and currently in phase I of clinical study
| Test Item | Test Method | |
| Appearance | Visual inspection | |
| Identification | Identification 1 | Restriction mapping |
| Identification 2 | Sanger sequencing | |
| Test | pH | Method 0631 of ChP 2020 |
| Purity | High performance liquid chromatography (HPLC) | |
| Residual E.coli host cell protein | ELISA | |
| Residual E.coli DNA | q-PCR | |
| Residual E.coli RNA | q-PCR | |
Residual antibiotics | ELISA | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Sterility | Method 1101 of ChP 2020 | |
| Concentration determination | DNA concentration | Method 0401 of ChP 2020 |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

As your project advances to pivotal clinical stages, we scale up and optimize processes to ensure the supply of large-volume, highly consistent plasmid materials for global multi-center Phase II/III trials. We focus on inter-batch quality stability and reproducibility, implementing stricter release standards to guarantee medication safety and supply continuity throughout long-term clinical studies, thereby providing solid data support for market approval.
| CDMO Services for Plasmids | ||||
| Types | Services | |||
| Clinical grade | 1 | GMP Manufacturing of Plasmids | ● Production output: 10 mg~1 g (subject to customized changes) ● Fermentation volume: 3~30 L (subject to customized changes) ● Purification method: three-step approach/two-step approach | ● Full-GMP workshop ● Separate workshops within non-sterile and sterile areas ● GMP quality management system ● Validated plant, facility and equipment compliant with clinical requirements |
| 2 | Technology Transfer | ● Technology transfer ● Receiving technology transfer | ● Well-established plan for technology transfer ● Well-established plan for receiving technology transfer ● Plan for transferring of different technologies across different phases | |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Advantages of our plasmid system:
• An independently developed four-plasmid system
• A system with the capability of sustained optimization
• Plasmid sequences are traceable, compliant with requirements, and efficient
• Extensive experience in successful IND submissions
• TCR-T cell samples for clinical use are currently manufacturing and in use
• 2-5 folds higher titers after using our plasmid system from the comparison in several projects
Advantages of our plasmid manufacturing:
• Free of antibiotics throughout the manufacturing process
• Plasmid production and bank creation in separate workshops
• Complete isolation between non-sterile and sterile areas
• Dispensing final products using an isolator
• Completed CTD dossiers for packaging plasmid (for lentiviral vector), reducing the submission preparation time by 3-4 months, with INDs of a few products granted preliminary approval and currently in phase I of clinical study

| Test Item | Test Method | |
| Appearance | Visual inspection | |
| Identification | Identification 1 | Restriction mapping |
| Identification 2 | Sanger sequencing | |
| Test | pH | Method 0631 of ChP 2020 |
| Purity | High performance liquid chromatography (HPLC) | |
| Residual E.coli host cell protein | ELISA | |
| Residual E.coli DNA | q-PCR | |
| Residual E.coli RNA | q-PCR | |
Residual antibiotics | ELISA | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Sterility | Method 1101 of ChP 2020 | |
| Concentration determination | DNA concentration | Method 0401 of ChP 2020 |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.

Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.

| CDMO Services for Plasmids | ||||
| Types | Services | |||
| Commercial grade | 1 | GMP Manufacturing of Plasmids | ● Production output: 10 mg~1 g (subject to customized changes) ● Fermentation volume: 3~30 L (subject to customized changes) ● Purification method: three-step approach/two-step approach | / |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages of our plasmid system:
• An independently developed four-plasmid system
• A system with the capability of sustained optimization
• Plasmid sequences are traceable, compliant with requirements, and efficient
• Extensive experience in successful IND submissions
• TCR-T cell samples for clinical use are currently manufacturing and in use
• 2-5 folds higher titers after using our plasmid system from the comparison in several projects
Advantages of our plasmid manufacturing:
• Free of antibiotics throughout the manufacturing process
• Plasmid production and bank creation in separate workshops
• Complete isolation between non-sterile and sterile areas
• Dispensing final products using an isolator
• Completed CTD dossiers for packaging plasmid (for lentiviral vector), reducing the submission preparation time by 3-4 months, with INDs of a few products granted preliminary approval and currently in phase I of clinical study

| Test Item | Test Method | |
| Appearance | Visual inspection | |
| Identification | Identification 1 | Restriction mapping |
| Identification 2 | Sanger sequencing | |
| Test | pH | Method 0631 of ChP 2020 |
| Purity | High performance liquid chromatography (HPLC) | |
| Residual E.coli host cell protein | ELISA | |
| Residual E.coli DNA | q-PCR | |
| Residual E.coli RNA | q-PCR | |
Residual antibiotics | ELISA | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Sterility | Method 1101 of ChP 2020 | |
| Concentration determination | DNA concentration | Method 0401 of ChP 2020 |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.

Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.
