Lentivirus, a subtype of retrovirus, can integrate the target gene into the host cell genome, and is commonly used as a viral vector for ex vivo cell engineering. With the emergence of cellular therapy industry, the market demands for lentiviral vectors are also increasing with each passing year. Hillgene is specialized in provision of integrated CDMO solutions for cellular therapy products, has established an advanced GMP grade platform for serum-free suspension culturing of lentiviral vectors, and therefore, can provide high-quality CDMO services for lentiviral vectors to clients with various demands.
Services
| CDMO Services for Lentiviral Vectors (HiLenti® Platform) | ||||
| Types | Services | |||
IIT grade | 1 | Independently developed four-plasmid system | ● Third generation four-plasmid system ● Kanamycin-resistance gene ● No patent license required | ● Seamless connection to IND submission |
| 2 | Manufacturing and Testing of Lentiviral Vectors (GMP-like) | ● Tailorable production output and specification | ● GMP-like workshop ● Authentic and traceable documentation ● GMP-like quality management system | |
*Note: We offer relatively flexible and customized changes to above services, including but not limited to above services.
Advantages
| Advantages of using our platform for serum-free suspension culturing of lentiviral vectors: • Free of animal-derived components throughout the process • Linearly scaled up production of lentiviral vectors • Using a single container of a 50 L disposable bioreactor • Cell bank creation in separate workshops • Dispensing final products using a sterile isolator • Dedicated lentivirus system for CAR-NK cells, with high infection efficiency • Low production costs and testing costs (no requirements of testing for BSA and residual pancreatic enzymes) • Several successful IND submissions to NMPA of lentiviral vectors for CAR-NK cells |
Manufacturing process

Quality control
| Product | Test Item | Test Method |
| Harvest Fluid | Adventitious virus contamination | Method 3302 of ChP 2020 |
| Replication-competent lentiviruses | Indicator cell culture method | |
| Drug substance/finished product | Appearance | Visual inspection |
| Sterility | Method 1101 of ChP 2020 | |
| Mycoplasma | Method 3301 of ChP 2020 | |
| pH | Method 0631 of ChP 2020 | |
| Osmolality | Method 0632 of ChP 2020 | |
| Target gene structure identification | Sequencing | |
| Residual host cell protein | ELISA | |
| Physical titer (p24) | ELISA | |
| Functional titer | Flow cytometry | |
| Endotoxin | Method 1143 of ChP 2020 | |
| Residual Benzonase | ELISA | |
| Residual host cell DNA | q-PCR | |
| Residual E1A gene transfer | Co-culture method | |
| Residual SV40 gene transfer | Co-culture method |
*Note: Hillgene established QC methods corresponding to different technology platforms, with QC methods including but not limited to above items.
Project Timeline

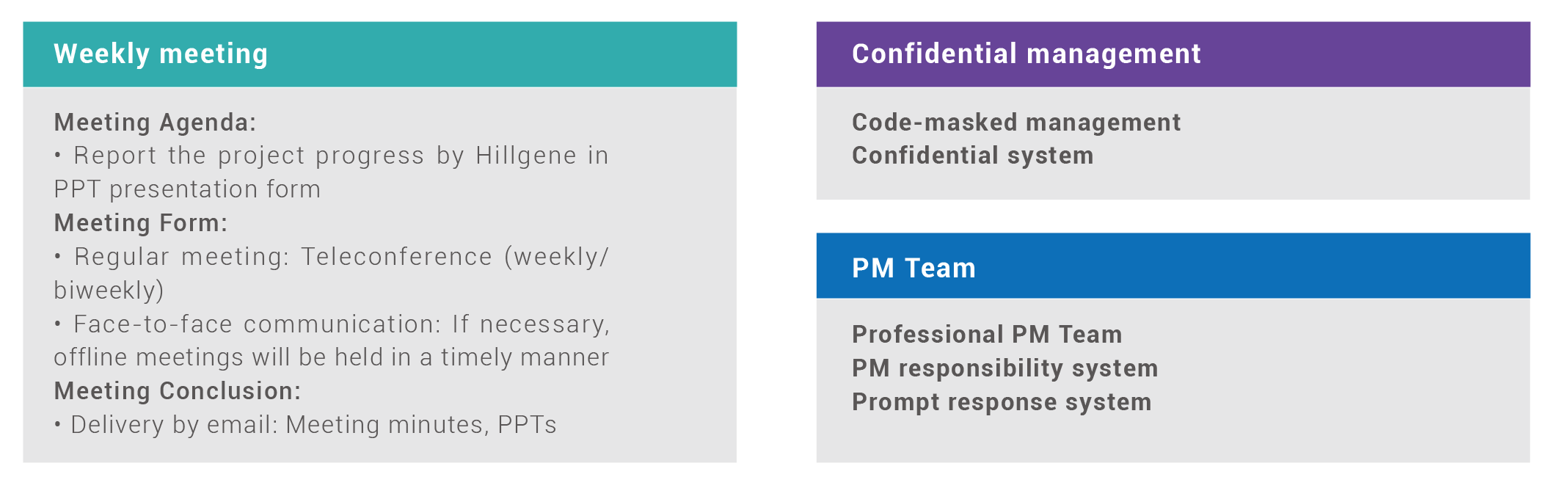
Project Management Plan
Hillgene Project Management Team, consisting of chief scientists, project managers, Project QA and GMP experts, will make efforts to ensure the smooth and sound operation of each and every GMP project.





Building 4, Yuewang Wisdom Valley, 1463 Wuzhong Avenue, Wuzhong District, Suzhou, China